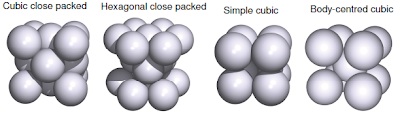
In this section we will see the solid structure of metals and their ionic compounds of s - and p - block elements. Besides that, we will also see the lattice enthalpy and the enthalpy of dissolution of ionic compounds, as well their periodic trends. Solid State Structures of Metals In general, the metallic elements would arrange itself in metallic lattice in the most efficient was in a fixed volumes. Then, it is assume that atoms as hard spheres and all atoms have identical size. From those assumption, there are 4 main solid state structures of metals; cubic close packed (CCP), hexagonal close packed (HCP), simple cubic, and body-centred cubic. The elements will arrange in certain crystal lattice depends on its atomic (metallic) radius, and most of metals are CCP or HCP. Metallic solid structures