Amphotericin B
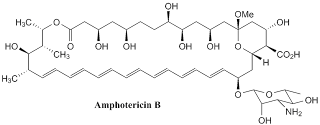
It was orginally extracted from a bacterium Streptomyces nodosus in 1955 at the Squibb Institute of Medical Research. This compound is called amphotericin B and now it is listed on the World Health Organisation's List of Essential Medicine , which is the list of the most important medications needed in basic health. Amphotericin B works as antifungal drug and is often used intravenously for systemic fungal infections. The name of amphotericin B is derived from its amphoteric property.