A Survey of the s- and p- Block Elements
In this section we will have a look for a brief survey of the s- and p-block elements. We will have a look a certain properties of the elements across the group and as well their trends for some properties.
Hydrogen
Hydrogen is known as the most abundant element in the universe and the 15th most abundant element on earth. The main properties of hydrogen gas are it is a colourless gas as well highly combustible gas, it forms diatomic molecular H2, and it has low melting and boiling point (m.p.=14 K and b.p.=20 K). The low melting and boiling point is caused by weak van der Waals interaction due to small size of H2 molecule. Besides that, the bond dissociation energy for H-H is 436 kJ mol−1. The position of H in periodic table can be debatable because it can be place in group 1 or group 17 with argument H can form H+ and H-. In nature, H exists in 3 different isotopes, H-1 is the most common one (almost 99.9% abundance of H), H-2 or deuterium which the deuterium compound is used for solvents in NMR spectroscopy, and H-3 or tritium which is radioactive with half life of around 12 year and it decay into He-3. Moreover, H is predicted to show metallic behaviour at very high pressure condition, so it is believed that Jupiter have a metallic core of hydrogen.Hydrogen gas can be manufactured in very different way as shown below (more detail see hydrogen fuel economy).
Steam reforming and coal gasification. Hydrogen gas can be synthesised by reacting methane or coal with water vapour in high temperature to form hydrogen gas and carbon monoxide. Then, to increase the yield of hydrogen gas synthesis, CO is reacted with water vapour to form hydrogen gas and carbon dioxide.
Dehydrogenation process is involved the hydrocarbon compound (e.g. ethane) to form double bond compound (e.g. ethene) by releasing hydrogen gas.
Electrolysis of water also produces hydrogen gas at cathode, meanwhile oxygen gas is formed in anode.
Hydrogen gas is very common in industrial process and main uses of hydrogen gas is in ammonia industry, and as well it is the common reducing agent. The uses of hydrogen gas is shown below.
 |
| The uses of hydrogen gas |
S-Block Metals
All the group 1 metals have body centre cubic (BCC) structure which is not a close-packed strucuture and it only has 1 valence electron per atom. Therefore, the delocalised electron sea in metallic structure of group 1 is less dense than group 2, which makes the melting point of group 1 is lower than group 2. Because group 2 has relatively smaller than group 1, it can exhibit more efficient packing, so it has close-packed structure (HCP [hexagonal close-packed] and CCP [cubic close-packed]). Moreover, the melting points decreases as down the group because of the metallic bonding becomes weaker as the metallic radius increases. For your information, d-block (transition) metals has more varies mixture of CCP, HCP, BCC, lattice structures.
The reactivity of group 1 metals is dominated by formation of the +1 oxidation state and the reactivity increases down group as the ionisation energy decreases. It means the energy to remove electron become smaller due to bigger radius which means weaker interaction between the valence electron as well the shielding effect of inner-core electrons which decreases the interaction. The reactions of group 1 metals are shown below.
Besides that, group 1 metal for example can have chemical properties as a solution in liquid ammonia (b.p. ammonia -33°C). When, alkali metals in liquid ammonia it forms identical colour for different electropositive metals (for example Na forms deep blue solution) and there is no hydrogen gas evolved. The coloured solution is due to solvated electron in the solution, and higher metal concentration gives bronze solution due to electrons delocalised throughout solution.
Moreover, solutions of alkali metals in liquid ammonia is electrically conducting and it is paramagnetic (unpaired electrons); Na in liquid ammonia is known as a good reducing agent (decomposition in presence of Fe2O3 gives NaNH2 + H2).
In group 2, it is dominated by formation of the +2 oxidation state (e.g. halides MX2, oxide MO, and peroxide MO2,). In the other sides, Be has special case due to very small ionic radius (59 pm), so its ionic compounds often contain hydrated cation, e.g. [Be(H2O)4]Cl2. Moreover, anhydrous BeCl2 has a polymeric chain structure with ionic/covalent character and the structure is similar to BeH2 but is not electron deficient as Cl has lone pair.
P-block elements
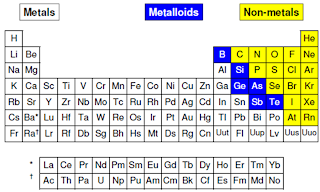
The structures of p-block elements is varied from metallic structures into only monoatomic molecules (group 18 elements). Besides that, there are also elements can exhibit covalent polyatomic molecule or network which are mainly metalloid elements; and as well the diatomic molecules (N, O, and group 17).From the structure as we see on figure above, the structural variation leads to irregular trends in group 13-16. In the other sides, the melting point of group 17 and 18 increase as down the group due to stronger intermolecular forces (van der Waals interaction). In p-block elements there are some structural features of p-block elements which are:
- Allotropy. Elements which can exist in more than one (structural) form in the same state exhibit allotropy and the different forms are called allotropes. The chemical bonding between atoms is different, with different discrete structural or molecular units. For example: C, Sn, P, O, and S.
- Polymorphism. Element or compounds which can exist in different crystal form but contain identical repeating units exhibit polymorphism and the different forms are called polymorphs. For example: rhombic and monoclinic sulphur, both contain S8 molecules.
- Catenation. Elements or compounds which form structures containing sequences of element-element bonds exhibit catenation. For example C as element or in alkanes.
Group 13 elements
 |
| B12 icosahedron (left) and part of one layer of the infinite lattice of α-rhombohedral boron. |
 |
| The construction of B84 unit, the main building block of β-rhombohedral B. (a) In the centre of the unit is B12 icosahedron, and to each these, another B atom is covalently bonded. (c) A B60-cage is the outer 'skin' of B84-unit. (d) The final B84-unit can be described in terms of covalently bonded subunits (B12)(B12)(B60) |
Group 14
 |
| Diamond crystal structure |
 |
| Graphite |
 |
| graphene |
 |
| buckminsterefullerene |
 |
| Fullerenes solutions in toluene |
Group 15 (The Pnictogens)
Nitrogen forms dinitrogen (N2) which has triple bond with bond energy of 946 kJ mol−1. Nitrogen gas is a colourless gas and the most abundant gas in the atmosphere (78%) with melting point of 63 K and boiling point of 77 K. |
| White phosphorus |
 |
| Black phosphorus |
The argument behind this different structural form of P and N can be seen from energetical sides or from the structural itself. From the structure, N has smaller atomic radius, so it has better overlap of p-orbital to form π-bonds than P. Besides that, it also can be seen from the thermochemical sides by using Hess's law. Hess's law states that the standard enthalpy of an overall reaction is the sum of the standard enthalpies of the individual reactions into which a reaction may be devided. This is the law upon which thermochemical (e.g. Born-Haber) cycles are based. Therefore for N and P are shown below.
 |
| Born-Haber cycle |
For heavier elements in group 15, As, Sb, Bi all have room temperature solid state structures resembling black phosphorus. The separation between layers decreases down the group and its behaviour becomes more metallic as As and Sb are metalloids and Bi is metallic. High pressure induces increased metallic character, As4 and Sb4 molecules exist in the vapour phase.
Group 16 (The Chalcogens)
 |
| Oxygen allotropes |
 |
| Sn |
 |
| Allotropy and polymorphism of sulphur |
Group 17 (the Halogens) and Group 18 (the Noble Gases)
All elements in group 17 exist as diatomic molecules, X2. F-F bonds is anomalously weak because it has short bond and lone pairs electrons repel each other and it makes F2 highly reactive. Besides that, I2 crystals have metallic lustre and it can conduct electric current at high region (iodine is close to metalloid region) and the solid of I2 is sublimes. The melting point and boiling point increases as down the group due to stronger van der Waals interaction, and the the bond enthalpy decreases as down the group due to increasing in atomic radius creates less efficient overlap which means weaker covalent bonding.Meanwhile, group 18 elements are all monoatomic gas and it solidifies at low temperature with close-packed crystal lattice. In general, group 18 elements are inert but heavier elements (Xe and Kr) form some compounds. The main use of group 18 elements is as a light sources such as neon lamps. Moreover, liquid He is used as a cooling agent of magnet in LHC (Large Hadron Collider) and some instrument such as NMR spectoscopy.







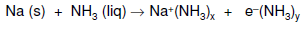





Comments