In this section, we will have a discussion around the drugs delivery and tissue engineering. In discussion about drugs delivery, we will see not only the topical methods, but also how to control the release of active pharmaceutics components. Lastly, we will see about tissue engineering with focus on the scaffolding.
To begin with, we should see the fact that the delivery and formulation is also important as the drug discovery and many pharmaceutics company spend huge amounts on drugs delivery. Besides that, around 1000 organic compounds are synthesised in the lab but they are useless as drugs because they cannot be administrated. Moreover, a drug is not a drug unless it can reach the target. There are certain main administration of drugs such as lotions and creams, pills, sprays, liquid injection, liquid injestion, eye drops, etc.
The routes to delivery drugs is important, as the topical-substance is applied at site of action. Besides that, systemic-substance applied generally enteral (via the digestive tract) and parenteral (via routes other than digestive tract). Meanwhile, topical-substances are applied:
- epicutaneous. Application onto the skin.
- inhalational.
- enema. Application into the anus.
- eye drops
- ear drops
- intranasal route. Into the nose.
- vaginal
The first topical drugs delivery is creams. In general, a cream is an emulsion of an oil in water and in this topic it means the therapeutics sit in the oil. Moreover, the therapeutics are covered with a nano-shell of polymer which has a function as stabiliser.
 |
| The diagram of creams drug |
Besides that, a creams formulation also used an emulsifying agent to ensure it forms a phase and the most common emulsifying agents are a long chain alkyl group with polar head group (e.g. carboxylic acids or esters).
 |
| Examples of emulsifying agent |
The second topical formulation is spray which is used as intranasal delivery and this method has 2 main advantages which are rapid adsorption and needle-free. This successful of this method depends on the charge and the size of molecules. Large charged particles are more difficult for permeation, so smaller and uncharged particles are easier for permeation. However, the size of the particles should be bigger than 10 μm otherwise they travel to the lungs. Moreover, pH should be matched with pH and pKa of formulation (for the best comfort range pH 4.5-6.5) and the molecular weight should less than 1000 g mol−1 for good diffusion.
 |
| The diagram how spray works |
Next topical formulation is pills or tablets which comprise of many components. The first component is active pharmaceutical ingredient, i.e. the therapeutic compound. Secondly is the binders or excipients which are an inert compound used for binding the formulation together. The binders hold the tablet together giving strength and the common binder such as starch, cellulose, modified cellulose, sugars, PEG, or PVP. Moreover, there are two main types of binders, solution binding which is dissolved in either water or alcohol and used wet during compounding. The second binder is dry binding which means drug and binder are mixed dry and moulded.
 |
| Solution binding (left) and examples of binder (centre and right) |
The third component is disintegrant which is a material that rapidly hydrates releasing the therapeutic. There are a lot of ways how disintegrant work to release the therapeutic. The first type of disintegrant is the capillary action and it is always the first step. In this mode, water penetrates pores and replaces air pushing the grains apart and disintegrant works by providing a low energy (hydrophilic) wettable surface.
 |
| Capillary action of disintegrant |
The next type of action is swelling and there are 2 types of swelling disintegrant modes. First one is wicking where the water is pulled into pores by disintegrant and reduce the physical bonding forces between particles. The other one is swelling where the particles swell and break up the matrix from within; swelling sets up; localised stress spreads throughout the matrix.
 |
| Swelling disintegrant |
Besides that, disintegrant also used the enthalpy to disintegrate the drug, this is called heat of wetting. In this mode, the enthalpy is released as water adsorps to pores, so the local temperature increases. The increasing temperature causes the air expands and the tablet is blown apart.
 |
| Heat of wetting disintegrant |
Another mode by using disintegrating particles or particles repulsive force. This mode is supported as the tablet is formed from the particles that are charged. Then, as the water penetrates, charge repulsion force separates particles. Another similar mode is called deformation where the particles are compressed in manufacture and the compressive force released on swelling.
 |
| Disintegrating particles and deformation |
The next disintegrant mode is applied on the effervescent tablets by releasing the gas. Basically, this tablet is made from sodium bicarbonate or carbonate mixed with acid such as tartaric acid or citric acid, and they are compounded dry then react when wet. The reaction between carbonate or bicarbonate with acid form carbon dioxide which disintegrate the tablet.
The last mode of disintegrant by employing the bodies enzymes to degrade the binder, for example:
- amylase for starch,
- protease for gelatin,
- cellulase for cellulose or modified cellulose,
- invertase for sucrose.
Another component of pills is lubricant which is generally needed to release the tablet from the mould. The last component is coating which is generally a polymer plus pigment and maybe plastisizer. The coating is used to aid printing, extending shelf life, improving taste, improving as and aid for swallowing.
The controlled release of drugs is also important as well because it determines the intake of drugs for the patient. In conventional system, after the drug is disintegrated all the drugs are released in one shot. Therefore, there are some consequences such as antibiotics which should be taken 5 pills per day, anti-malarials for a pills everyday for up to 2 months, contraceptive pills in humans for daily, and diabetic patients take regular insulin injections.
 |
| The schematic diagram of conventional drug release system |
However, in alternative release of drugs such as slow release the drugs is released either by diffusion or degradation not in one shot until it is exhausted.
 |
| The schematic diagram of alternative slow release drug system |
There are many types how to release drugs slowly and the first types is drug in matrix. The drugs is mixed in with matrix and the matrix is sometimes a degrading polymer or a swollen polymer such as hydrogel. For degrading polymer matrix, the drugs is released as the polymer degrades and the degradation from surface as the first drug is released as polymer degrades. Meanwhile, in hydrogel matrix the drug diffuses out over time and the hydrogel restrict diffusion.
 |
| Drug in matrix |
Another similar types is by using a reservoirs, such as capsules. The drug is dispersed or dissolved in another medium and coated with a permeable or degrading membrane. Hence, the drug diffuses through the membrane.
 |
| Drug reservoir |
The polymer particles is also used to control the drug release with various physical methods for incorporating drugs, e.g. pan coating, air-suspension coating, spray drying etc. An example of chemical method is the interfacial polymerisation of the nylon rope trick. The drugs trapped in particle with diamine molecule and the surrounding is diacyl chloride, so the polymer film forms at the interface.
 |
| Chemical method |
The last example of controlled release is the polymer drugs that is used for cancer treatment. The drugs is linked by a degradable linker on the water soluble polymer and the polymer is also attached a targeting group, the drug is inactive while attached to the polymer. As the targeting group is binds to cell, the linker degrades and releases drugs into the target.
 |
| Polymer drugs |
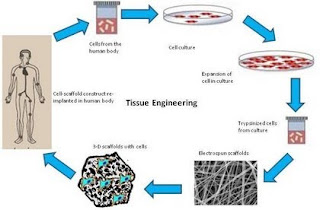
After we see how the drugs are delivered, the next part is one of the solutions for global issue which is the demand for transplant organ cannot be satisfied. Therefore, regenerative medicine plays its role as the concept of repairing tissue with cells rather than artificial materials which is known as tissue engineering. In tissue engineering the cells are cultured into tissue usually on, in, or around a scaffold with plus several variants such as in vivo, in vitro, transfer, responsive removal, etc. In this section we will in vitro and in vivo variants.
 |
| General scheme of tissue engineering |
In vitro strategy means the cells are grown outside the body by using a degradable scaffold. There are 3 ways of in vitro strategy after the cells are seeded in biodegradable scaffold and the cells adhere, proliferate, and form tissue. The first strategy is directly implant these cells with the scaffold or the cells removed by for example temperature change and then implant it. The third way where the scaffold degrades, so the cell can be implanted.
 |
| In vitro tissue engineering |
Meanwhile, in vivo strategy as the cells are seeded in a scaffold, there are 2 ways to do in vivo strategy. In vivo strategy means inside the body or in this term is the scaffold degrades inside the body. The first way is the cells are recruited in vivo, so this way is encouraged the cell regeneration. The second way is the cells adhere, proliferate, form tissue, and then implant it. Therefore, the scaffold degrades inside the body.
 |
| In vivo tissue engineering |
There are a lot of types of current biodegradable polymers for scaffolds, such as:
 |
| example of scaffolds |
- PLG/PCL [poly(lactic acid co-glycolic acid)/polycaprolactone]. These polymers are amorphous with high rate of degradation.
- Polyurethane
- Poly(ether-ester)
- Peptide polymers
- Polyanhydrides/polycarbonates
- Polysaccharides, e.g. chitosan.
- Polyphosphonate.
 |
| Peptide polymer degradation |
The peptide polymer scaffold was pioneered by Hubbel et al which is the polymer as a hydrogel. The main chain of this polymer contain peptides, so it can be cleaved by MMP (Matrix metalloproteinase) enzymes. The advantage of this method is very specific and controllable oligomers, so it is less toxic than low molecular weight polymers. However, the disadvantages are very expensive, hard to make large quantities, and need to discover other suitable peptides.
 |
| Chitosan (left) and hyaluronic acid (right) |
Another common scaffold is polysaccharides and the most important scaffolds are chitosan and hyaluronic acid. These scaffolds need modification before they can be processed and generally need modification for cell adhesion. Moreover, they have a good record in vivo-low toxicity degradation products and another example of polysaccharides scaffold is alginates.
 |
| Alganites |




















Comments