Organic Chemistry for A-Level: Benzene and Its Derivates
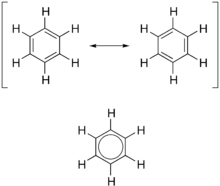
A. Structure The structure of benzene was suggested firstly by Kekulé as a cyclic molecule but he also thought benzene had alternative single and double carbon-carbon bonds. The Kekulé structure is expected that benzene undergoes addition reaction. However, benzene is far less easily to undergo addition reaction than alkene, such as cyclohexene. Besides that, the carbon-carbon bond lengths of benzene are all identical, with the lengths intermediate between those single and double bonds.