Compounds of s- and p- Block Elements
In this section, we will have a survey in elements of s- and p- block elements. Since, the elements of s- and p- block is varies, we only have a look for binary compound of hydrogen, oxide, and halides; as well we will see the trends in the periodic table.
Group 1 ionic hydrides form general formula is MH and it is formed by reacting the metal with hydrogen gas; the structure of MH (e.g. NaH) is the rock- salt (NaCl) structure. Meanwhile, group 2 metals except Be and Mg form MH2. This ionic hydrides have characteristic which are:
Moreover, although it forms H−, the ionic hydrides is stable due to large lattice enthalpy.
Be, Mg, and Al form polymeric hydrides and in general it is hydride compound that forms from small, and polarising cation. Because it has a small and highly dense-charged ions, it can induce covalent character, so it is not purely ionic because electrons of hydride anion are attracted towards small cation.
For example is BeH2, in gas phase it forms linear molecule but in solid state it forms chain structure with tetrahedral Be and bridging H atoms. The interesting point from this structure is the amount of electron is not sufficient to form this structure, so it is called electron deficient compound which implies its instability.
In group 13, B and Ga, form dimeric molecular compounds (M2H6) with the same structure as BeH2 and many larger boron hydrides also exist, known as boranes. Moreover, Ga2H6 has similar structure but less stable. AlH3 forms polymeric structure due to its electronegativity in the border of metalloid and it volatile solid with octahedrally coordinated Al. Heavier elements of In ad Tl hydrides is not stable due to weaker bonds with H. Besides that, group 13 hydrides anions has electron precise structure of anions EH4- (e.g. sodium borohydride NaBH4 and lithium aluminium hydride LiAlH4). These compounds are commonly used as reducing agents in organic reactions.
Group 14 binary hydrides form electron-precise structure which means it has correct number of electron pairs to draw a lewis structure with no lone pairs on central atom. Prominent hydride compounds in group 14 are carbon and silicon hydrides. Carbon hydrides can form long chain of hydrides compounds which is known as alkanes. Meanwhile, silicon hydride (silane) chain compound is thermally unstable as it releases hydrogen gas. Silane ignite spontaneously on contact with air and stability of group 14 hydrides decreases as down the group.
Group 15-17 forms electron-rich structure which means it has more electron pairs than are needed for bond formation; non-bonding (lone) pairs remain on the central atom. This structure can form relatively strong intermolecular force which is known as hydrogen-bonding interaction. H-bonding found in compounds of H with the more electronegative elements. Polar E-H bond leads to intermolecular interaction between H(δ+) and lone pair on E(δ-) and it influences structures and physical properties. The physical properties of the compounds that can exhibit hydrogen-bonding will have higher boiling point than another element in its group. Meanwhile, for structure it can be seen in solid water (ice) which forms open structure that makes ice less dense than liquid water.
In p-block elements, E-H covalent bond strengths decrease down the group due to poorer overlap of small H 1s orbital with valence shell orbitals of larger E, better orbital size-match. Meanwhile, across the period it becomes more stronger the E-H covalent bond due to highly electronegative elements which polarised more the E-H bond (ionic contribution).
Besides that, the acidity of p-block hydride increases down each group due to weaker E-H bond and more dispersed negative charge in anion formed by loss of proton, more delocalisation of negative charge in larger anion. Furthermore, the acidity increases from left to right due to increasing electronegativity, polar E-H bond more acidic.
The figure below is to summarise the structure and stability of binary hydride compounds of s- and p-block elements.
S- and p-block elements can exhibit the highest oxidation state to form fluoride compounds. The group 1 and 2 metals (except Be) forms ionic compounds due to its very low electronegativity. Meanwhile Be and group 13 (except B), and heavier elements of group 14 and 15 (Sn, Pb, Sb, and Bi) elements form polymeric structure due to its electronegativity in the borderline of metal/metalloid. Moreover, the rest of p-block fluoride compounds are molecular covalent; heavier elements of group 18 (Kr and Xe) can form fluoride compounds (KrF2 and XeF6). The high electronegativity of F can induce high oxidation compound or even form compounds with group 18. The period 2 fluoride compounds cannot exceed the octet rule as the heavier elements can exceed that rule as this is caused by 2s/2p orbitals accomodate only 8 electrons. Meanwhile, heavier elements can exceed this rule as a hypervalent compounds and those compounds are favoured by electronegative partner (e.g F, O) as the d-orbitals can involved in this compounds. Moreover, high oxidation state compounds are stabilised by small size of F which favours high coordination number.
For chloride compounds, group 1, group 2 (except Be), and heavier elements of group 13 (In and Tl) form ionic compound due to low electronegativity. Increasing electronegativity into the borderline elements can form polymeric structure such as Al, Ga, In, and heavier elements of group 14, 16, and 17 (Sn, Pb, Se, Te, and I). The rest of p-block elements form molecular covalent and it still form high oxidation state. However, the chlorides molecular covalent compounds has lower oxidation state compare to fluorides as Cl has lower electronegativity than F. In chlorides compound we see that the heavier p-block elements has a tendency to form compound with low oxidation states (n-2), where n is the maximum oxidation state for the group. This is caused as the bond strengths decreases down the group, and for example of Tl and Pb, the 6s electrons are very strongly held by nucleus and reluctant to be shared, which is known as the inert pair effect.
Group 13 p-block halides can have lewis acid behaviour as it has trigonal planar and boron sp2 hybridised. Boron halides form adducts with lewis bases as amines (NR3), phosphines (PR3), ethers (OR2), and halides (X-). This acid-base interaction can also describe the formation of dimer AlCl3 (Al2Cl6). Furthermore, AlCl3 forms polymeric lattice in solid state and forms dimer in liquid and gas phases.
Group 18 elements can form halide compounds as the ionisation energy and electronegativity decreases as descend the group, meaning that compound formation is more likely for the heavier noble gases (the valence electrons is being more available to share in covalent bonds). Xe (and to a lesser extent Kr) is able to form compounds with very electronegative elements such as F and O.
The fluorides of Xe ( XeF2, XeF4, and XeF6) can be made by combination of the elements under photochemical conditions, but the products are very reactive and act as fluorinating and oxidising agent. The oxide XeO3 and mixed oxyfluoride, XeOF4 formed by the reaction of XeF6 with water are also highly reactive, with Xe formally in the +6 oxidation state. Kr will not form compounds so readily but KrF2 is known.
The oxyanions of some p-block elements are shown above and these are the anions derived from the oxyacids such as carbonic acid (H2CO3), nitric acid (HNO3), sulphuric acid (H2SO4), phosphoric acid (H3PO4), and perchloric acid (HClO4). Moreover, the acidic hydrogens in these species are connected to the element E via an oxygen (i.e. the H is in an OH group); there is no E-H bond.
In group 14 and 15 the lightest elements forms a small molecular oxides, e.g. diatomics and triatomics, involving multiple bonding. π-bonds between O and C or N are strong due the good size-match of p-orbitals. For heavier elements π-bonds to O are not so strong and single bonds are preferred. Hence, despite the similar empirical formulae, SiO2 (silica, quartz) has a very different structure to CO2, with an extended network of Si-O single bonds. The phosporus oxides shown adopt cage structures where each has a tetrahedron of 4 P atoms (as in white phosporus P4) but linked by 6 bridging oxygens. Besides that, P4O10 has four additional terminal oxygens.
Binary compounds of hydrogen
The s- and p-block elements can form binary compound with hydrogen and as the characteristic of the elements varies, the types of compounds are also different. Group 1 and 2 metals form ionic (saline or salt like) compound with the hydrogen has -1 charge (H−), with exception of Be and Mg. The ionic hydrides is a compounds with electropositive s-block metals, so it can make hydrogen to form H−.Group 1 ionic hydrides form general formula is MH and it is formed by reacting the metal with hydrogen gas; the structure of MH (e.g. NaH) is the rock- salt (NaCl) structure. Meanwhile, group 2 metals except Be and Mg form MH2. This ionic hydrides have characteristic which are:
- non-volatile crystalline solids
- insoluble in common non-aqueous solvents
- electrically non-conducting solids but conduct in molten state
- in electrolysis, it forms H2 at anode
Moreover, although it forms H−, the ionic hydrides is stable due to large lattice enthalpy.
Be, Mg, and Al form polymeric hydrides and in general it is hydride compound that forms from small, and polarising cation. Because it has a small and highly dense-charged ions, it can induce covalent character, so it is not purely ionic because electrons of hydride anion are attracted towards small cation.
For example is BeH2, in gas phase it forms linear molecule but in solid state it forms chain structure with tetrahedral Be and bridging H atoms. The interesting point from this structure is the amount of electron is not sufficient to form this structure, so it is called electron deficient compound which implies its instability.
In group 13, B and Ga, form dimeric molecular compounds (M2H6) with the same structure as BeH2 and many larger boron hydrides also exist, known as boranes. Moreover, Ga2H6 has similar structure but less stable. AlH3 forms polymeric structure due to its electronegativity in the border of metalloid and it volatile solid with octahedrally coordinated Al. Heavier elements of In ad Tl hydrides is not stable due to weaker bonds with H. Besides that, group 13 hydrides anions has electron precise structure of anions EH4- (e.g. sodium borohydride NaBH4 and lithium aluminium hydride LiAlH4). These compounds are commonly used as reducing agents in organic reactions.
Group 14 binary hydrides form electron-precise structure which means it has correct number of electron pairs to draw a lewis structure with no lone pairs on central atom. Prominent hydride compounds in group 14 are carbon and silicon hydrides. Carbon hydrides can form long chain of hydrides compounds which is known as alkanes. Meanwhile, silicon hydride (silane) chain compound is thermally unstable as it releases hydrogen gas. Silane ignite spontaneously on contact with air and stability of group 14 hydrides decreases as down the group.
Group 15-17 forms electron-rich structure which means it has more electron pairs than are needed for bond formation; non-bonding (lone) pairs remain on the central atom. This structure can form relatively strong intermolecular force which is known as hydrogen-bonding interaction. H-bonding found in compounds of H with the more electronegative elements. Polar E-H bond leads to intermolecular interaction between H(δ+) and lone pair on E(δ-) and it influences structures and physical properties. The physical properties of the compounds that can exhibit hydrogen-bonding will have higher boiling point than another element in its group. Meanwhile, for structure it can be seen in solid water (ice) which forms open structure that makes ice less dense than liquid water.
In p-block elements, E-H covalent bond strengths decrease down the group due to poorer overlap of small H 1s orbital with valence shell orbitals of larger E, better orbital size-match. Meanwhile, across the period it becomes more stronger the E-H covalent bond due to highly electronegative elements which polarised more the E-H bond (ionic contribution).
Besides that, the acidity of p-block hydride increases down each group due to weaker E-H bond and more dispersed negative charge in anion formed by loss of proton, more delocalisation of negative charge in larger anion. Furthermore, the acidity increases from left to right due to increasing electronegativity, polar E-H bond more acidic.
The figure below is to summarise the structure and stability of binary hydride compounds of s- and p-block elements.
Halides
This part we will have a look in fluorides and chlorides compounds as general consideration.S- and p-block elements can exhibit the highest oxidation state to form fluoride compounds. The group 1 and 2 metals (except Be) forms ionic compounds due to its very low electronegativity. Meanwhile Be and group 13 (except B), and heavier elements of group 14 and 15 (Sn, Pb, Sb, and Bi) elements form polymeric structure due to its electronegativity in the borderline of metal/metalloid. Moreover, the rest of p-block fluoride compounds are molecular covalent; heavier elements of group 18 (Kr and Xe) can form fluoride compounds (KrF2 and XeF6). The high electronegativity of F can induce high oxidation compound or even form compounds with group 18. The period 2 fluoride compounds cannot exceed the octet rule as the heavier elements can exceed that rule as this is caused by 2s/2p orbitals accomodate only 8 electrons. Meanwhile, heavier elements can exceed this rule as a hypervalent compounds and those compounds are favoured by electronegative partner (e.g F, O) as the d-orbitals can involved in this compounds. Moreover, high oxidation state compounds are stabilised by small size of F which favours high coordination number.
For chloride compounds, group 1, group 2 (except Be), and heavier elements of group 13 (In and Tl) form ionic compound due to low electronegativity. Increasing electronegativity into the borderline elements can form polymeric structure such as Al, Ga, In, and heavier elements of group 14, 16, and 17 (Sn, Pb, Se, Te, and I). The rest of p-block elements form molecular covalent and it still form high oxidation state. However, the chlorides molecular covalent compounds has lower oxidation state compare to fluorides as Cl has lower electronegativity than F. In chlorides compound we see that the heavier p-block elements has a tendency to form compound with low oxidation states (n-2), where n is the maximum oxidation state for the group. This is caused as the bond strengths decreases down the group, and for example of Tl and Pb, the 6s electrons are very strongly held by nucleus and reluctant to be shared, which is known as the inert pair effect.
Group 13 p-block halides can have lewis acid behaviour as it has trigonal planar and boron sp2 hybridised. Boron halides form adducts with lewis bases as amines (NR3), phosphines (PR3), ethers (OR2), and halides (X-). This acid-base interaction can also describe the formation of dimer AlCl3 (Al2Cl6). Furthermore, AlCl3 forms polymeric lattice in solid state and forms dimer in liquid and gas phases.
Group 18 elements can form halide compounds as the ionisation energy and electronegativity decreases as descend the group, meaning that compound formation is more likely for the heavier noble gases (the valence electrons is being more available to share in covalent bonds). Xe (and to a lesser extent Kr) is able to form compounds with very electronegative elements such as F and O.
The fluorides of Xe ( XeF2, XeF4, and XeF6) can be made by combination of the elements under photochemical conditions, but the products are very reactive and act as fluorinating and oxidising agent. The oxide XeO3 and mixed oxyfluoride, XeOF4 formed by the reaction of XeF6 with water are also highly reactive, with Xe formally in the +6 oxidation state. Kr will not form compounds so readily but KrF2 is known.
Oxides of p-block elements
The lighter and more electronegative elements tend to form molecular oxides whereas the heavier less electronegative elements form polymeric or ionic lattices, and this is similar to the trend found for p-block halides. Moreover, the oxides of p-block elements can form oxyanions as a result of oxyacid deprotonation,In group 14 and 15 the lightest elements forms a small molecular oxides, e.g. diatomics and triatomics, involving multiple bonding. π-bonds between O and C or N are strong due the good size-match of p-orbitals. For heavier elements π-bonds to O are not so strong and single bonds are preferred. Hence, despite the similar empirical formulae, SiO2 (silica, quartz) has a very different structure to CO2, with an extended network of Si-O single bonds. The phosporus oxides shown adopt cage structures where each has a tetrahedron of 4 P atoms (as in white phosporus P4) but linked by 6 bridging oxygens. Besides that, P4O10 has four additional terminal oxygens.
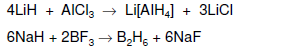




















Comments