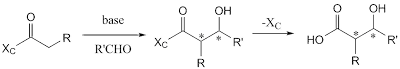
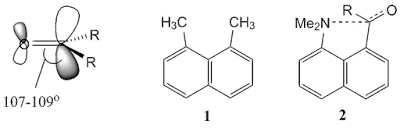
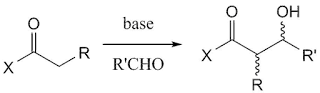Ecteinascidin 743

One of the main attractions in the chemistry of natural products, apart from facing new challenges in synthetic methodologies, is discovering a novel therapeutic agent such as ecteinascidin 743 (ET-743) . This compound was isolated for the first time as pure compound from Caribbean tunicate Ecteinascidia turbinata in 1986 by Rinehart's group. This compound possess interesting properties as a potent anti-tumour agent and ET-743 is approved in Europe, Russia and South Korea for treatment of advanced soft tissue sarcoma. From synthetic chemists' point of view, ET-743 provides an interesting challenge as it has interesting molecular architecture; comprised of eight rings, including 10-membered heterocycles and 8 stereogenic centres. This interesting molecular architecture and interesting anti-tumour properties were the motivation of its first total synthesis by E. J. Corey in 1996.