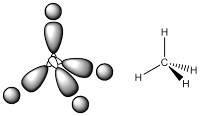
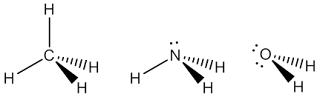
In this section, we will have a discussion about the covalent bonding in a molecule from two point of view, hybridisation and molecular orbital (MO) theory. In this section we will have a discussion about the hybridisation related to some fundamental geometries and in MO theory, we will see how to construct a simple MO for diatomic molecule. This section is a continuity from previous discussion about VSEPR (see A Quick Guide to VSEPR ). From VSEPR calculations help to predict shape of molecules but tell us nothing about the bonding. Once we know the shape of a molecule, we can think about how the atoms are held together, as the nature of bonding, and there is more than one way to do this. The first point of view is the bonding as the localised models, a bond involves overlap of two orbitals on adjacent atoms (hybridisation). In the other hands, the molecular bonding can be seen as delocalised models, a bond involves orbitals spread over the whole molecule (MO theory).