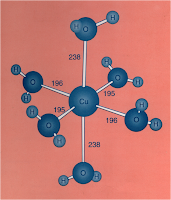
Entropy: The 2nd and 3rd Laws of Thermodynamics

In this section, we will have a discussion about the origin of spontaneity of process by looking some of the thermodynamics properties, one of it is entropy. Besides that, we will also see the thermodynamics laws that become the foundations of the concept of entropy which are the 2nd and 3rd laws of thermodynamics. Furthermore, we will see one of the application of the 2nd law of thermodynamics in Carnot engine. An idealised (impossible) engine

.JPG)