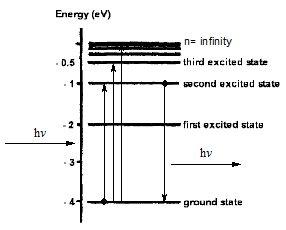
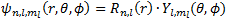Before we discuss more thoroughly about the H atom as a wave function, it might be better to have a look the older atomic model. Firstly, the Rutherford's model was not completely incorrect about the structure. Its model was built from its observation when a gold foil was shot by alpha particle. Rutherford's model only stated that electron is orbiting a nucleus, so the interaction is purely electrostatic interaction. However, the weakness of this model is the collapsing orbit of an electron. Since an electron is attracted by force from nucleus, so it is accelerating motion, and as the energy as the result of movement increases, it will collapse to the nucleus. Fortunately, this phenomena does not happen. Moreover, this structure also does not agree with the observation at the spectra. In this model it is expected to have a broad region of spectra, but in reality it appears as the line spectra.