The Chemistry of d- and f- Block: Complex Synthesis and Stability Constant
In this section, we will have a discussion how to make a complex compound through some common methods or reactions such as substitution or redox reaction. Besides that, we will also see some thermodynamics and kinetics aspect in complexation reaction, one of the thermodynamics parameter which will be discussed is the stability constant, K. Lastly, we will also see the pattern of K in complexation reaction with some abnormal cases.
The most common way to make a complex compound is by using substitution where one or more ligands is replaced by different one. This reaction is often done in aqueous solution,
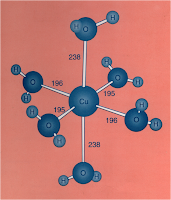
Besides that, the analogue of substitution reaction in aqueous solution is what actually happens in the solution itself. For example, in aqueous [Ni(OH2)6]2+, the complex is surrounded by water molecules and these are called the outer sphere waters. Meanwhile, the water molecules that is bonded to metal ions as the ligands are called the inner sphere waters, and the substitution reaction between outer sphere and inner sphere waters happens very fast.
A word of warning when complexes is isolated from its solution sometimes can cause problem as the isolated complexes are not always the same as the species in solution as demonstrated below.
In isolating the complexes through crystallisation, it can be influenced by the counter ion when it has two possible products. For example in the reaction between [Ni(OH2)6]2+ and KCN, it produces [Ni(CN)5]3- and [Ni(CN)4]2-, and the problem comes when it comes to isolate one of the product. This product can be isolated by using different counter ion, because [Ni(CN)4]2- is relatively smaller anion than [Ni(CN)5]3-, so smaller cation such as K+ can be used to isolate it. Meanwhile, [Ni(CN)5]3- is isolated by using bigger cation, such as another complex cation [Cr(en)3]3+ to form [Cr(en)3][Ni(CN)5]. As a further note, big anion and big cation (or small anion and small cation) are favourable together and matching the charge could also influence the crystallisation.
The substitution reaction could also be carried out in the non-aqueous solvents because of the property of ligands such as in the reaction between and bipy. This reaction is carried out in the ethanol because bipy is insoluble in water [Fe(OH2)6]3+.
In this reaction, if the reaction under aqueous solution, the water molecules drive the reaction backward as the reaction is reversible.
The other terminologies that are commonly used in complex synthesis is anation and aquation. Anation means a ligands substitution reaction when neutral ligands is replaced by an anion.
Meanwhile aquation is the ligand substitution reaction when any other ligands are replaced by water molecule.
The other ways of making complexes is by direct reaction which the precursor compound is reacted with the ligands.
In those reactions, there is no ligand displacement. Another reaction is using the redox reaction which there is change in oxidation number of metal ions.
In the reaction above, the oxidation states of cobalt is change from 2 into 3 [Co(II) to (Co(III)].
When it comes to the synthesis of a compound, there are 2 parameters that become the point of attention, the thermodynamics and kinetics. The thermodynamics parameter is a measure of the extent to which a particular species will form from other species when the system has reached equilibrium. The parameter that is used in thermodynamics in the case of complex compound reaction is the equilibrium constant K and this tells us about the position of equilibrium, but this does not tell us anything about how fast the reaction will go.
The kinetics parameter refers to the speed at which the transformation of leading to attainment of equilibrium will occur and the parameter is called rate constant k and this tell us about the rate of reaction.
Let's put this case into a problem. For example we have a reaction between A + B that can produce either C + D or F + G which has different reaction profile. The reaction A + B to produce F + G has smaller activation energy [EA(2)] barrier than the reaction to form C + D, so in room temperature F + G would be form faster than C + D. Hence, F and G is called the kinetic product and the reaction is reversible because the energy barrier is small. Meanwhile, an enormous energy barrier, EA(1), to form C + D is needed to overcome to give products C + D which has lower energy than F + G. We can say that C + D is thermodynamically more stable than F + G, so C + D is the thermodynamics products and the reaction to form C + D is irreversible due to huge energy barrier. Furthermore, the kinetics parameter is determined by how big the energy barrier to overcome while the thermodynamics measurement is determined from the enthalpy of reaction.
Firstly, the kinetics for the substitution reaction of [Fe(OH2)6]3+, the complexes can be divided into labile and inert. Labile complexes are the complexes that undergo rapid substitution reaction (e.g. within 60 seconds) and inert complexes are the complexes that undergo slow substitution reactions (e.g. minutes, hours, days, weeks or even years). Therefore, in a substitution reaction, a labile complex is used as the precursor compound and hopefully the product is an inert complex. In the first row transition series, the hexaquo complexes are mostly labile, only Cr(III) and Co(III) are inert.
The hexaquo metal cations has some acidity characteristic because when a water molecules is bonded as ligands, the O-H bond is weaken and this causes the acidity of a hexaquo complex. Therefore, dissolving some metal salts gives you an acidic solution.
Now, let consider a substitution reaction:
We can work out equilibrium constants, K, for all six steps:
Besides that, we can also work out the overall stability constant, β, as followed:
where β can be calculated from K as followed:
In those equations, shorthand notations are used as water molecules omitted, square brackets omitted, charges omitted so M means [M(OH2)6]n+ and [M] means the concentration of that ion.
When we consider a reaction
In the first step of this reaction there will be x water molecules that we can replace by a ligand L. Meanwhile, in the last step of this reaction there will be one water molecule that we can replace by a ligand L. Therefore, we have to think about the probability of substitution.
Now, let consider step y of that reaction:
This step will have equilibrium constant Ky and the relative probability of the forward reaction will be proportional to:
Then, when we consider step y+1 of that reaction:
This step will have equilibrium constant Ky+1 and the relative probability of the forward reaction this time will be proportional to:
Therefore, we have two proportionalities so we can make a ratio:
which gives us a purely statistical ratio of K values for each step. Furthermore, in the table below is the comparison between theoretical and experimental ratios.
However, the pattern does not always show the same where in some cases shows some abnormality and the abnormality is caused by certain factors as followed.
The first factor is the geometry change occurs at the substitution reaction as in the example of Fe(III)/Cl- system where K4/K3 is abnormally large.
In this example, the geometry changes from octahedral to tetrahedral and the reaction is also driven by increasing in entropy; the same case is also happened in Cd(II)/Cl- system. Moreover, similarly low values of of K5 and K6 observed for square planar d8 complexes. The geometry change also occurs because of Jahn-Teller distortions for example in Cu(II)/NH3 system and for comparison with Ni(II)/NH3 system as shown below.
The K5 and K6 in Cu(II)/NH3 system is extremely low, even K6 cannot be measured, and this is caused by one of is NH3 weakly bonded due to tetragonal elongation so it can be replaced easily by water molecules. Furthermore, to synthesis [Cu(OH2)6]2+ can be done by reacting anhydrous Cu(II) with liquid NH3.
The second factors is from the steric hindrance of the ligands as demonstrated on the table below.
From the schematic reaction below, we can see that it is more difficult to confine a large group of R and R' in the octahedral complex and this difficulty leads to higher ratio of K2/K3.
Furthermore, 2,2'-bipyridyl usually forms complexes for [M(bipy)3]n+ most metals but by adding a methyl group to make bipy becomes 6,6'-dimethyl-2,2'-bipyridyl causes difficulty to get three of these ligands round a metal.
The third factor is the change in electronic structure occurs in the substitution reaction as demonstrated in Cr(II)/bipy system.
The change in electronic structure of Cr(II) complex is simply caused by the fact that bipy is a stronger field ligand than OH2. At the first substitution, a bipy ligand substitutes 2 water molecules and because bipy is a stronger field ligand it causes the Δo becomes bigger. At the second substitution, because the Δo is much bigger than the pairing energy, it changes the electronic structure from high spin to low spin. Another example is in Fe(II)/bipy complex where the change in electronic structure happens in third step.
The most common way to make a complex compound is by using substitution where one or more ligands is replaced by different one. This reaction is often done in aqueous solution,
Besides that, the analogue of substitution reaction in aqueous solution is what actually happens in the solution itself. For example, in aqueous [Ni(OH2)6]2+, the complex is surrounded by water molecules and these are called the outer sphere waters. Meanwhile, the water molecules that is bonded to metal ions as the ligands are called the inner sphere waters, and the substitution reaction between outer sphere and inner sphere waters happens very fast.
 |
| What happens in aqueous solution |
A word of warning when complexes is isolated from its solution sometimes can cause problem as the isolated complexes are not always the same as the species in solution as demonstrated below.
 |
| A word of warning! |
 |
| Influencing crystallisation |
The substitution reaction could also be carried out in the non-aqueous solvents because of the property of ligands such as in the reaction between and bipy. This reaction is carried out in the ethanol because bipy is insoluble in water [Fe(OH2)6]3+.
 |
| Synthesis of a complex under non-aqueous condition |
The other terminologies that are commonly used in complex synthesis is anation and aquation. Anation means a ligands substitution reaction when neutral ligands is replaced by an anion.
Meanwhile aquation is the ligand substitution reaction when any other ligands are replaced by water molecule.
The other ways of making complexes is by direct reaction which the precursor compound is reacted with the ligands.
In those reactions, there is no ligand displacement. Another reaction is using the redox reaction which there is change in oxidation number of metal ions.
In the reaction above, the oxidation states of cobalt is change from 2 into 3 [Co(II) to (Co(III)].
When it comes to the synthesis of a compound, there are 2 parameters that become the point of attention, the thermodynamics and kinetics. The thermodynamics parameter is a measure of the extent to which a particular species will form from other species when the system has reached equilibrium. The parameter that is used in thermodynamics in the case of complex compound reaction is the equilibrium constant K and this tells us about the position of equilibrium, but this does not tell us anything about how fast the reaction will go.
The kinetics parameter refers to the speed at which the transformation of leading to attainment of equilibrium will occur and the parameter is called rate constant k and this tell us about the rate of reaction.
 |
| A reaction profile |
Let's put this case into a problem. For example we have a reaction between A + B that can produce either C + D or F + G which has different reaction profile. The reaction A + B to produce F + G has smaller activation energy [EA(2)] barrier than the reaction to form C + D, so in room temperature F + G would be form faster than C + D. Hence, F and G is called the kinetic product and the reaction is reversible because the energy barrier is small. Meanwhile, an enormous energy barrier, EA(1), to form C + D is needed to overcome to give products C + D which has lower energy than F + G. We can say that C + D is thermodynamically more stable than F + G, so C + D is the thermodynamics products and the reaction to form C + D is irreversible due to huge energy barrier. Furthermore, the kinetics parameter is determined by how big the energy barrier to overcome while the thermodynamics measurement is determined from the enthalpy of reaction.
 |
| I must no mix up kinetics and thermodynamics |
Firstly, the kinetics for the substitution reaction of [Fe(OH2)6]3+, the complexes can be divided into labile and inert. Labile complexes are the complexes that undergo rapid substitution reaction (e.g. within 60 seconds) and inert complexes are the complexes that undergo slow substitution reactions (e.g. minutes, hours, days, weeks or even years). Therefore, in a substitution reaction, a labile complex is used as the precursor compound and hopefully the product is an inert complex. In the first row transition series, the hexaquo complexes are mostly labile, only Cr(III) and Co(III) are inert.
The hexaquo metal cations has some acidity characteristic because when a water molecules is bonded as ligands, the O-H bond is weaken and this causes the acidity of a hexaquo complex. Therefore, dissolving some metal salts gives you an acidic solution.
Now, let consider a substitution reaction:
We can work out equilibrium constants, K, for all six steps:
Besides that, we can also work out the overall stability constant, β, as followed:
where β can be calculated from K as followed:
In those equations, shorthand notations are used as water molecules omitted, square brackets omitted, charges omitted so M means [M(OH2)6]n+ and [M] means the concentration of that ion.
When we consider a reaction
In the first step of this reaction there will be x water molecules that we can replace by a ligand L. Meanwhile, in the last step of this reaction there will be one water molecule that we can replace by a ligand L. Therefore, we have to think about the probability of substitution.
Now, let consider step y of that reaction:
This step will have equilibrium constant Ky and the relative probability of the forward reaction will be proportional to:
Then, when we consider step y+1 of that reaction:
This step will have equilibrium constant Ky+1 and the relative probability of the forward reaction this time will be proportional to:
Therefore, we have two proportionalities so we can make a ratio:
which gives us a purely statistical ratio of K values for each step. Furthermore, in the table below is the comparison between theoretical and experimental ratios.
However, the pattern does not always show the same where in some cases shows some abnormality and the abnormality is caused by certain factors as followed.
The first factor is the geometry change occurs at the substitution reaction as in the example of Fe(III)/Cl- system where K4/K3 is abnormally large.
In this example, the geometry changes from octahedral to tetrahedral and the reaction is also driven by increasing in entropy; the same case is also happened in Cd(II)/Cl- system. Moreover, similarly low values of of K5 and K6 observed for square planar d8 complexes. The geometry change also occurs because of Jahn-Teller distortions for example in Cu(II)/NH3 system and for comparison with Ni(II)/NH3 system as shown below.
 |
| The log K of Cu(II)/NH3 system and Ni(II)/NH3 system |
The second factors is from the steric hindrance of the ligands as demonstrated on the table below.
From the schematic reaction below, we can see that it is more difficult to confine a large group of R and R' in the octahedral complex and this difficulty leads to higher ratio of K2/K3.
 |
| The steric hindrance effect |
The third factor is the change in electronic structure occurs in the substitution reaction as demonstrated in Cr(II)/bipy system.
 |
| The change in electronic structure effect in Cr(II)/bipy system |














Comments