The Chemistry of d- and f- Block: Trends in Stability Constant and The Application of Complex Chemistry
In this section, we will see the trends in stability constant where the ligands and the metal ions are changed. We will see some patterns that build up this concept about the trends and end up in hard and soft acid base theory. Lastly, in this section we will see how complex chemistry is used in our daily life.
Firstly, when we want to predict the trend of stability constant, we need to ask can we see any trends in K values for a particular ligand when we change metal ions? The first concept of this trend is the Irving-Williams series where for a given ligand, the stability of complexes with usually follows the order:
The other trend is Ahrland, Chatt and Davies series which in this series the metal ions are classified into two groups depending on the trends in K values of their halide complexes.
From those series, it comes to the Pearson's Hard and Soft acids and bases (HSAB) theory and Pearson suggested that class 'a' should be called hard and class 'b' is soft. However, HSAB theory has a much wider application than just metal complexes; it can be applied to all Lewis acids and bases. This series was done by using a reaction:
This reaction allows us to determine relative affinity of base B for H+ or CH3Hg+. In this reaction, if K is big so base B is a soft one; while if K is smaller than 1 so base B is a hard one. Furthermore, this experiment is very dangerous. From the experiment above, we get the results as shown below.
Now, what actually the data tell us? From the table, F, O and N donors clearly prefer the hard acid H+ while Cl, Br, I, S and P prefer the soft acid CH3Hg+. Hence, we can conclude that hard acids prefer hard bases and soft acids prefer soft bases. Moreover, hard acids have properties such as small radius, not polarisable, and highly charged while soft acids are larger, and not so highly charged. Meanwhile, hard bases are small, not highly polarisable, and not highly charged while soft bases are larger and more polarisable.
This HSAB theory also has an impact to ligands characteristic which is the ambidentate ligands. An ambidentate ligand is a ligand with two possible points of attachment such as thiocyanate SCN-. The ligand binds to hard metal centres through N and binds to soft metal centres through S. Besides that, it can sometimes observe examples where presence of hard ligands can metal more hard, and presence of soft ligands can make it more soft.
Furthermore, HSAB theory can be seen by using Klopman's MO analysis which is the separated charge-charge interactions and orbital-orbital interactions. Hard acid-hard base interactions are charge controlled (i.e. ionic) whereas soft acid-soft base interactions are frontier molecular orbital controlled (i.e. covalent).
However, HSAB theory is not 100% correct which one of the exceptions is BeCl2. is the most covalent of the group 2 chlorides; it sublimes, and also dissolves in organic solvents. Based on HSAB, it implies that Be2+ should be the hardest cation in the group and should form the most ionic compounds. However, Beryllium small size and highly charged means it is the most polarising and this distorts the electron clouds of the chloride ions, resulting a more covalent structure.
From all concepts that we met in previous parts of chemistry of d- and f-block, we will see how it can be applied in daily life. Firstly, the complex chemistry is commonly used in analytical chemistry where it can be use qualitatively or quantitatively. The most common example is the reaction between Fe(III) and thiocyanate, and the reaction of dimethylglyoxime (DMGH2) with Ni(II).
The reaction of Fe(III) with thiocyanate produces a deep red coloured solution while the reaction between Ni(II) with produce DMGH2 red precipitate.
Secondly, our concept of HSAB is commonly used in metal extraction and with this theory we can predict the occurence of the metal ores. The first class of mineral is lithophiles which found in earth's crust in association with oxide (a hard base), so this ores would contain hard acids such as Li, Mg, Ti, Si, etc. The second class is chalcophiles which found in combination with sulphide ( a soft base), so this ores would contain soft acids such as Cd, Pb, Bi, Sb, etc. Furthermore, the borderline acids can be found as both.
The complexation reaction is also used in metal extraction for example Cu, and this method is called hydrometallurgy.
The copper complex is formed in organic layer with a rigid structure due to internal hydrogen bonding interaction. Because the reaction is reversible, adding acids in the complex solution would drive the reaction back to form Cu(II) ion which can be isolated in aqueous layer. Complex reaction is also used when extracting noble metals of gold and silver with cyanide ions.
Another example of metal extraction using complex reaction is isolation of Ni by Mond's process. This reaction involves CO as the ligand to form [Ni(CO)4], which is volatile and highly toxic, and heat it again to isolate Ni metal.
Ligands of hard bases are commonly used in detergents to trap the that causes hard water. The ligands such as polyphosphates, EDTA or NTA is used in detergents to trap the metal ions.
Furthermore, crown ethers is also used because its selectivity with alkali and alkaline earth metals. The crown ethers are hard donor atoms with cyclic structure and this enhance the stability of the complex because of the macrocyclic effect. The selectivity of crown ether comes from its ring size which allows crown ether to be selective binding agent with group 1 and 2 metal ions.
Metal complex is also in MRI as the contrast agent and the metal ion that is used is Gd(III). However, to act as the contrast agent Gd(III) needs one or more H2O ligands attached but [Gd(OH2)n]3+ (n = 8, 9) is highly toxic because it is the same size as Ca2+ and competes with it in enzymes. Therefore, to overcome this problem a strongly bound ligands which allow Gd to be excreted after use is needed, and the ligands are shown below.
The complexes formed are very stable because they have lots of chelate rings and Gd(III) is a hard metal ion and ligands have hard O and N donors. The remaining water molecule is labile and exchange rapidly with outer sphere waters so every second lots of water molecules 'see' the gadolinium.
When we talk about metal, sometimes there are concerns about the toxicity. However, sometimes this can lead to an ambiguity, are we talking about the toxicity of the metal or its compounds? Besides that, how toxic is toxic? Generally, everything is toxic in large enough amounts. There are some metals which are toxic such as beryllium, cadmium and mercury.
Beryllium is formerly used in fluorescent light phosphorus; also used in allys with Cu and Ni, and in nuclear reactors and X-ray tubes. All compounds and the metal are extremely toxic, probably due to replacing Mg in key enzymes.
Meanwhile, Cd is used in coatings, alloys and batteries; and its compounds and the metal is extremely toxic because it binds ot -SH groups in enzymes and also replaces Zn. Furthermore, it also causes kidney failure even in very small amounts.
Another common toxic metal is Hg which is used in chlor-alkali process for Cl2 and NaOH, amalgam formation, thermometer, etc. The metal is very toxic by inhalation and Hg(II) compounds are very toxic, methylmercury compounds are incredibly toxic. The target of this compounds is the central nervous system and HgNO3 used in making felt hats, leading to 'hatter's shakes' and 'mad as a hatter'.
One of the famous cases of dimethyl mercury when Karen Wetterhahn spilt a drop or 2 drops of HgMe2 on her glove in the fume cupboard and within a few months she started getting neurological symptoms. The tests showed she had 80 times the toxic level of mercury in her blood and within less than a year after the spillage she was dead.
A former ingredients of rat poison which is thallium salts and all forms of thallium are very toxic by skin contact, inhalation, and ingestion. Thallium targets central nervous system, and also causes characteristic hair loss.
Another famous toxic metal is lead which is used in batteries, ammunition, lead weights, and roofing; lead was formerly used in piping, paints, petrol additives, etc. The lead compounds are cumulative poisons which causes nausea, anaemia, kidney failure, brain damage, CNS disorder, etc. Another toxic metal is arsenic and all As compounds are toxic. In 2010, about 77 million people was exposed to As-contaminated drinking water in Bangladesh, according to a Lancet study (source: BBC).
When we are accidentally exposed with the unwated or toxic metal ions, it can be removed by using chelation therapy. This method involves complex reaction and this was originated at the Great War to remove chemical weapon such as lewisite which contain As. This compound has a characteristic smell of geranium and it binds with -SH groups at the enzymes. Therefore, the British sides developed anti-lewisite which can counteract the effect of lewisite. The design of anti-lewisite basically based on the fact that As is a soft acid, so it requires soft bases such as -SH functional group.
However, the challenge in this therapy is to design ligands that specifically remove the only unwanted metals. The examples are Na2CaEDTA is used for lead poisoning, desferrioxamine B for Fe, or D-penicillamine for Hg, Pb and Cu poisoning.
Besides that, metals also play prominent roles in biology as shown on the table below.
In general, there are 2 main biological ligands which are porphyrin which forms -2 charged ligand and corrin which forms -1 charged ligand.
One of the famous porphyrin ligand in biological system is used in haemoglobin to bind the oxygen. In haemoglobin, porphyrin ring makes a complex with Fe(II) and this complex is high spin. When it binds with oxygen, it changes into the low spin which causes the change in conformation of haemoglobin to allow the other oxygen is bound within the haemoglobin and this is known as cooperative binding. The globin protein has a function to protect Fe(II) from oxidation reaction.
Meanwhile, an example of corrin complex is in vitamin B12 which is a Co(III) complex with R group. In nature the R group is CH3 while in most of the isolation it turns out to be CN. The study of vitamin B12 is quite sophisticated, so to study this compound the analogue compounds could be used.
Another complex compound property that is used in daily life is the trans effect in Cl and NH3 ligands which allows us to synthesis cisplatin. The trans effect is an effect that causes the ligands in trans position to another ligand becomes labile. From this fact, we can decide which precursor compound that can be used to synthesis cis-platin and the synthesis is shown below.
By using the same scheme, we can synthesis trans-platin with different precursor compound.
Firstly, when we want to predict the trend of stability constant, we need to ask can we see any trends in K values for a particular ligand when we change metal ions? The first concept of this trend is the Irving-Williams series where for a given ligand, the stability of complexes with usually follows the order:
Ba2+ < Sr2+ < Ca2+< Mg2+< Mn2+< Fe2+< Co2+< Ni2+< Cu2+> Zn2+This series is due to a combination of electrostatic effects (i.e. radius of ion) and ligand field effects. In this series, the decreasing radius of ion would increase stability because it related closely to the splitting energy stabilisation.
The other trend is Ahrland, Chatt and Davies series which in this series the metal ions are classified into two groups depending on the trends in K values of their halide complexes.
Class 'a' => F- > Cl- > Br- > I-Class 'b' => F- < Cl- < Br- < I-
From this series, it was found that the Class 'a' included Ti4+, Cr3+, alkali metals (group 1), and alkaline earth metals (group 2). Meanwhile, Class 'b' included Cu+, Ag+, Hg+, Hg2+, Pd2+ and Pt2+. Besides that, in this series, the ligands with first row donor atoms preferred class 'a' metals (e.g. N, O, F) whereas ligands with second or third row donor atoms preferred class 'b' metals (e.g. P, As, S, Se, Cl, Br, I).
From those series, it comes to the Pearson's Hard and Soft acids and bases (HSAB) theory and Pearson suggested that class 'a' should be called hard and class 'b' is soft. However, HSAB theory has a much wider application than just metal complexes; it can be applied to all Lewis acids and bases. This series was done by using a reaction:
This reaction allows us to determine relative affinity of base B for H+ or CH3Hg+. In this reaction, if K is big so base B is a soft one; while if K is smaller than 1 so base B is a hard one. Furthermore, this experiment is very dangerous. From the experiment above, we get the results as shown below.
Now, what actually the data tell us? From the table, F, O and N donors clearly prefer the hard acid H+ while Cl, Br, I, S and P prefer the soft acid CH3Hg+. Hence, we can conclude that hard acids prefer hard bases and soft acids prefer soft bases. Moreover, hard acids have properties such as small radius, not polarisable, and highly charged while soft acids are larger, and not so highly charged. Meanwhile, hard bases are small, not highly polarisable, and not highly charged while soft bases are larger and more polarisable.
This HSAB theory also has an impact to ligands characteristic which is the ambidentate ligands. An ambidentate ligand is a ligand with two possible points of attachment such as thiocyanate SCN-. The ligand binds to hard metal centres through N and binds to soft metal centres through S. Besides that, it can sometimes observe examples where presence of hard ligands can metal more hard, and presence of soft ligands can make it more soft.
Furthermore, HSAB theory can be seen by using Klopman's MO analysis which is the separated charge-charge interactions and orbital-orbital interactions. Hard acid-hard base interactions are charge controlled (i.e. ionic) whereas soft acid-soft base interactions are frontier molecular orbital controlled (i.e. covalent).
However, HSAB theory is not 100% correct which one of the exceptions is BeCl2. is the most covalent of the group 2 chlorides; it sublimes, and also dissolves in organic solvents. Based on HSAB, it implies that Be2+ should be the hardest cation in the group and should form the most ionic compounds. However, Beryllium small size and highly charged means it is the most polarising and this distorts the electron clouds of the chloride ions, resulting a more covalent structure.
From all concepts that we met in previous parts of chemistry of d- and f-block, we will see how it can be applied in daily life. Firstly, the complex chemistry is commonly used in analytical chemistry where it can be use qualitatively or quantitatively. The most common example is the reaction between Fe(III) and thiocyanate, and the reaction of dimethylglyoxime (DMGH2) with Ni(II).
 |
| Analytical chemistry based upon the complex compounds |
Secondly, our concept of HSAB is commonly used in metal extraction and with this theory we can predict the occurence of the metal ores. The first class of mineral is lithophiles which found in earth's crust in association with oxide (a hard base), so this ores would contain hard acids such as Li, Mg, Ti, Si, etc. The second class is chalcophiles which found in combination with sulphide ( a soft base), so this ores would contain soft acids such as Cd, Pb, Bi, Sb, etc. Furthermore, the borderline acids can be found as both.
The complexation reaction is also used in metal extraction for example Cu, and this method is called hydrometallurgy.
 |
| Hydrometallurgy |
Another example of metal extraction using complex reaction is isolation of Ni by Mond's process. This reaction involves CO as the ligand to form [Ni(CO)4], which is volatile and highly toxic, and heat it again to isolate Ni metal.
Ligands of hard bases are commonly used in detergents to trap the that causes hard water. The ligands such as polyphosphates, EDTA or NTA is used in detergents to trap the metal ions.
Furthermore, crown ethers is also used because its selectivity with alkali and alkaline earth metals. The crown ethers are hard donor atoms with cyclic structure and this enhance the stability of the complex because of the macrocyclic effect. The selectivity of crown ether comes from its ring size which allows crown ether to be selective binding agent with group 1 and 2 metal ions.
Metal complex is also in MRI as the contrast agent and the metal ion that is used is Gd(III). However, to act as the contrast agent Gd(III) needs one or more H2O ligands attached but [Gd(OH2)n]3+ (n = 8, 9) is highly toxic because it is the same size as Ca2+ and competes with it in enzymes. Therefore, to overcome this problem a strongly bound ligands which allow Gd to be excreted after use is needed, and the ligands are shown below.
The complexes formed are very stable because they have lots of chelate rings and Gd(III) is a hard metal ion and ligands have hard O and N donors. The remaining water molecule is labile and exchange rapidly with outer sphere waters so every second lots of water molecules 'see' the gadolinium.
When we talk about metal, sometimes there are concerns about the toxicity. However, sometimes this can lead to an ambiguity, are we talking about the toxicity of the metal or its compounds? Besides that, how toxic is toxic? Generally, everything is toxic in large enough amounts. There are some metals which are toxic such as beryllium, cadmium and mercury.
Beryllium is formerly used in fluorescent light phosphorus; also used in allys with Cu and Ni, and in nuclear reactors and X-ray tubes. All compounds and the metal are extremely toxic, probably due to replacing Mg in key enzymes.
Meanwhile, Cd is used in coatings, alloys and batteries; and its compounds and the metal is extremely toxic because it binds ot -SH groups in enzymes and also replaces Zn. Furthermore, it also causes kidney failure even in very small amounts.
Another common toxic metal is Hg which is used in chlor-alkali process for Cl2 and NaOH, amalgam formation, thermometer, etc. The metal is very toxic by inhalation and Hg(II) compounds are very toxic, methylmercury compounds are incredibly toxic. The target of this compounds is the central nervous system and HgNO3 used in making felt hats, leading to 'hatter's shakes' and 'mad as a hatter'.
One of the famous cases of dimethyl mercury when Karen Wetterhahn spilt a drop or 2 drops of HgMe2 on her glove in the fume cupboard and within a few months she started getting neurological symptoms. The tests showed she had 80 times the toxic level of mercury in her blood and within less than a year after the spillage she was dead.
A former ingredients of rat poison which is thallium salts and all forms of thallium are very toxic by skin contact, inhalation, and ingestion. Thallium targets central nervous system, and also causes characteristic hair loss.
Another famous toxic metal is lead which is used in batteries, ammunition, lead weights, and roofing; lead was formerly used in piping, paints, petrol additives, etc. The lead compounds are cumulative poisons which causes nausea, anaemia, kidney failure, brain damage, CNS disorder, etc. Another toxic metal is arsenic and all As compounds are toxic. In 2010, about 77 million people was exposed to As-contaminated drinking water in Bangladesh, according to a Lancet study (source: BBC).
When we are accidentally exposed with the unwated or toxic metal ions, it can be removed by using chelation therapy. This method involves complex reaction and this was originated at the Great War to remove chemical weapon such as lewisite which contain As. This compound has a characteristic smell of geranium and it binds with -SH groups at the enzymes. Therefore, the British sides developed anti-lewisite which can counteract the effect of lewisite. The design of anti-lewisite basically based on the fact that As is a soft acid, so it requires soft bases such as -SH functional group.
However, the challenge in this therapy is to design ligands that specifically remove the only unwanted metals. The examples are Na2CaEDTA is used for lead poisoning, desferrioxamine B for Fe, or D-penicillamine for Hg, Pb and Cu poisoning.
Besides that, metals also play prominent roles in biology as shown on the table below.
In general, there are 2 main biological ligands which are porphyrin which forms -2 charged ligand and corrin which forms -1 charged ligand.
One of the famous porphyrin ligand in biological system is used in haemoglobin to bind the oxygen. In haemoglobin, porphyrin ring makes a complex with Fe(II) and this complex is high spin. When it binds with oxygen, it changes into the low spin which causes the change in conformation of haemoglobin to allow the other oxygen is bound within the haemoglobin and this is known as cooperative binding. The globin protein has a function to protect Fe(II) from oxidation reaction.
 |
| The Haemoglobin in action |
Meanwhile, an example of corrin complex is in vitamin B12 which is a Co(III) complex with R group. In nature the R group is CH3 while in most of the isolation it turns out to be CN. The study of vitamin B12 is quite sophisticated, so to study this compound the analogue compounds could be used.
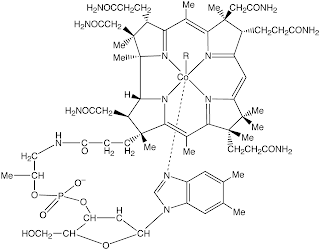 |
| Vitamin B12 |
Another complex compound property that is used in daily life is the trans effect in Cl and NH3 ligands which allows us to synthesis cisplatin. The trans effect is an effect that causes the ligands in trans position to another ligand becomes labile. From this fact, we can decide which precursor compound that can be used to synthesis cis-platin and the synthesis is shown below.
 |
| Cisplatin synthesis |
 |
| Transplatin synthesis |










Comments