Previously, in asymmetric aldol reaction a chiral enolate is used to ensure diastereoselective reaction by introducing more control due to chiral centre at enolate or carbonyl. This time, another method is used to synthesis an enantiopure product which is using chiral auxiliary functional group.
 |
| Chiral auxiliary-controlled aldol reaction |
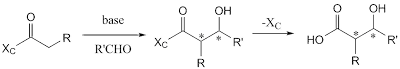
In this method, an enantiopure auxiliary (XC) is used and it carries out a diastereoselective reaction such as aldol reaction. Furthermore, the reaction provides enantiopure product after removal of the chiral auxiliary.
The first method in using chiral auxiliary is Evans aldol reaction where an oxazolidinone moiety is used as the chiral auxiliary group.
 |
| Evans asymmetric aldol reaction |
In this method, the auxiliary can be prepared from an enantiopure ethanolamine and dialkyl carbonate ester followed by attachment of the carbonyl compound.
 |
| Preparation of chiral auxiliary group |
The chiral auxiliary is employed to control enolate geometry and facial selectivity of enolate addition to the aldehyde.
The first step in Evans aldol reaction is the enolate formation using a strong Lewis acid (Bu2BOTf) and trialkylamine base (e.g. iPr2NEt).
 |
| Enolate formation |
The enolisation process occurs via 6-membered chair type transition state where the R group is pointing away from the chiral auxiliary group; hence,
Z-enolate is favoured in this process. Then, the facial selectivity of the aldehyde addition is dictated by the chiral auxiliary. However, there is a problem because there are two possible rotamers of this chiral auxiliary.
 |
| The addition of aldehyde |
In this case, only one rotamer of the intermediate is favoured where the electrostatic repulsion is minimised; hence, the rotamer with opposite dipole is favoured. This favoured intermediate implies that the addition can only happen from the front face. As an example, Evans aldol reaction is featured intensively in the
synthesis of natural product cytovaricin.
Furthermore, the auxiliary group is removed by using peroxide reagent such as LiOOH.
 |
| Removal of chiral auxiliary group |
Another variation in Evans aldol reaction is asymmetric alkylation. In this reaction, the enolate formation happens via 'open' transition state and the electrophile approaches away from Bn group.
 |
| Evans asymmetric alkylation |
The final product of Evans asymmetric aldol and alkylation reactions is carboxylic acid, so it needs further steps to form carbonyl or ketone. However, another chiral auxiliary group can be used in ketone alkylation which is Enders alkylation. In this method, an enantiopure (
S)-(-)/(
R)-(+)-1-amino-2-methoxy-methylpyrollidine is used as the auxiliary group.
 |
| Enders asymmetric alkylation reaction |
The enolisation process by LDA favours the formation
E-enolate rather than
Z-enolate and this is due to steric interactions in
Z-enolate between pyrollidine and R group.
 |
| (a) Enders alkylation mechanism and (b) Enders alkylation of aldehyde |
The addition of the electrophile can only be done from bottom face which is less steric interaction. The fused-ring intermediate is pointing upward which makes the top face is less accessible towards the electrophile. Besides that, Enders reaction also works with aldehydes as well.
The final examples of chiral auxiliary controlled reaction is the Schöllkoff asymmetric synthesis of α-amino acids. Amino acids can provide good chiral auxiliary group as out of 20 amino acids only one is achiral, glycine. In Schöllkoff reaction, the α-amino acids is synthesised from glycine and valine to give "chiral glycine equivalent". The "chiral equivalent" is deprotonated by LDA and reacted with electrophile. The attack of the electrophile happens to the front face, in this example, and this is due to steric interactions by valine side group which points inward.
 |
| Schöllkoff asymmetric synthesis of α-amino acids |
The synthesised amino acid can be isolated after hydrolysis reaction and separation; the products are methyl esters and methyl ester valine can be recovered for another reaction.










Comments