In nucleophilic addition in carbonyl compounds, it involves breaking π(C=O) bond by adding electrons into C=O antibonding orbitals. This implies the trajectory of attack for addition of a nucleophile follows the shape of π*(C=O) allowing proper overlap with the orbitals. The trajectory of this nucleophile is around 107-109° and this angle is called Bürgi-Dunitz angle; named after H. B. Bürgi and J. D. Dunitz who discovered the physical evidence that supports the this angle of attack (H. B. Bürgi, J. D. Dunitz, and E. Shefter,
J. Am. Chem. Soc., 1973,
95, 5065-5067).
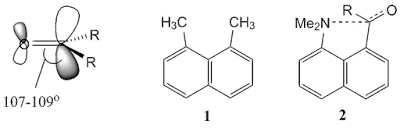 |
| Bürgi-Dunitz angle and the evidence of the trajectory of nucleophilic attack. |
Bringing this idea in carbonyl addition reaction, this reaction would generate a new chiral centre from the pro-chiral carbonyl compound. This implies a racemic mixture will be formed. However, when the carbonyl compound has a stereogenic centre next to C=O, it adds a new issue; a pair of diastereoisomers are formed.
 |
| Nucleophilic addition of chiral carbonyl |
The way to rationalise this reaction is by using Felkin-Anh model and normally C=O addition follows this model.
In this model, the preferred transition state orients the sterically dominant group, R
L anti to incoming nucleophile and perpendicular to C=O group to minimise the non-bonding interactions between the incoming nucleophile and R
L.
 |
| Felkin-Anh control in nucleophilic addition of chiral carbonyl |
Then, the nucleophile preferentially attacks C=O along the Bürgi-Dunitz path via the conformation which minimises non-bonding interactions with those substituents at the adjacent carbon. The destabilising interaction in the disfavoured transition state is the steric interaction between the nucleophile and R
M substituent. One of the example of the nucleophilic addition of carbonyl under Felkin-Anh control is aldol reaction using chiral aldehyde.
 |
| Felkin-Anh controlled aldol reaction of chiral aldehyde |
Apart from Felkin-Anh control, the stereocontrol of this addition can also have chelation control. This type control happens when one group on the α-carbon can chelate to the carbonyl via metal counterion.
 |
| Felkin-Anh chelation control |
Furthermore, Felkin-Anh control can also be applied in the absence of metal counterion where α-heteroatom is anti to the incoming nucleophile and perpendicular to C=O plane. This arrangement is due to minimisation of electrostatic repulsion between X and nucleophile.
 |
| Felkin-Anh control of non-chelating heteroatom reaction |






Comments