Storage and Transport of Iron in Living Things
Fe is known as the most abundant metal in the human body and it also comes with variety of functions such as haemoglobin (O2 transport in blood), myoglobin (O2 storage in muscle), cytochrome c (electron transfer), catalase (metabolism of H2O2), and ribonucleotide reductase (transformation of RNA to DNA).
However, there are two major problems associated with handling it in biological system. Firstly, in aqueous environment, the stable oxidation state of Fe is Fe(III) which precipitates as the insoluble hydrated oxide [FeO(OH)]n or rust. Besides that, free iron is also toxic as traces of high-spin Fe(II) generates superoxide radicals and hydroxyl radicals which are highly reactive and damage cells.
Therefore, whole process of Fe transport and storage keeps Fe under very close control; concentration of free iron in mammals is around 10-24 M.
In mammals, Fe from food is transported in blood by proteins called transferrins and it bounds Fe as shown below.
Every transferrin has two Fe-binding sites and Fe(III) bound strongly in 'hard' donor set with binding constant 1023 M-1 at pH 7.4. This condition prevents precipitation of Fe(III) as oxide. From the binding site, a synergestic binding with carbonate anion also present and this enhances Fe(III) binding. In the binding, carbonate anion organises the binding pocket by H-bonding interactions with arginine side chain, and two amide NH bonds gives correct octahedral conformation for Fe(III) binding to occur.
In the Fe transport, Fe(III)/transferrin complex moves across lipid membrane by endocytosis.
The mechanism of Fe transport into cell can be divided into 6 main steps. (1) The free transferrin, Apo-transferrin (Apo-Tf), binds to Fe ions to form Fe-transferrin complex (Fe-Tf). (2) Fe-Tf complex binds to membrane receptor TfR and then membrane starts to fold in forming a vesicle. (3) Lipid-based endosome is formed in which Fe-Tf is protected from cytoplasm. (4) After that, H+ ions are pumped into endosome to lower the pH from 7.4 to 5.5. (5) This influx protons, protonated tyrosine and aspartate which then reduces affinity to Fe(III). This Fe(IIII) then can be released from endosome via DMT1 channel that embedded in endosome membrane. (6) The regenerated Apo-Tf then is released from the endosome via exocytosis mechanism.
The key to controlling uptake and release Fe(III) is pH swing as phenolate and carboxylate bind Fe(III) strongly when deprotonated (pH 7.4) but not when protonated (pH 5.5).
Inside cell Fe is stored as ferritin until required, preventing undesired radical reactions. Ferritin consists of core of hydrated ferric oxide a protein overcoat. The protein overcoat is assembled from 24 protein units in roughly sphericla array.
Ferritin has inner diameter of 7.5 nm and outer diameter of 13 nm with the interior cavity accommodates up to 4500 Fe(III) ions. The snall gaps between the coat proteins provide both hydrophobic and hydrophilic channels for entry/exit of Fe ions. However, the exact form in which Fe enters ferritin is unknown, as is mechanism by which Fe is removed from core when needed, but likely to be as Fe(II).
Inside core the iron crystallises with OH- ions and dihydrogen-phosphate ions to form a crystal with approximate stoichiometry is [FeIII9O9(OH)8(H2PO4)]. The structure is similar to that of mineral ferrihydrite, 5Fe2O3.9H2O, common in earth's crust but with dihydrogenphosphate terminating groups on exterior surface which anchor the protein overcoat to the ferric oxide core.
All the Fe in human body and mammals are taken from their diet such as from meat or Fe-rich plants. On the other hand, lower organisms such as bacteria need to extract it from environment where it exists predominantly as insoluble minerals.
Bacteria excretes siderophores to strip Fe(III) from minerals and form soluble complexes which are absorbed by organism. They are the strongest known binders of Fe(III) and it is based on tris-catecholate or tris-hydroxamate ligands which gives octahedral Fe(III) complex.
Thus, combining three bidentate chelating sites in one molecule gives even stronger binding such as in enterobactin that is used by bacteria such E.coli to scavenge iron from environment.
The increase in binding affinity is due to enterobactin is a hexadentate ligand instead of three bidentate ligands. Besides that, preorganisation of ligand minimises cost of conformational change and increases binding affinity.
On the other hand, tris-hydroxamate siderophores such as ferrichrome (from) yeast and desferrioxamine give the same effect as in enterobactin. Ferrichrome is a cyclic hexapeptide with three chelating arms pendant from cyclic core and partly preorganised octahedral cavity for Fe(III).
Meanwhile, desferrioxamine is a linear tris-hydroxamate from bacteria and it folds up around Fe(III) centre to act as hexadentate ligand. It is also used to bind excess ion in case of Fe overload from either poisoning or disease.
As mentioned earlier, those ligands bind very strongly to Fe(III) so it gives a challenge for the release mechanism making Fe ions can be used for metabolism. Unaided release would be too slow to be biologically relevant, so there are three possibilities which may all occur for different system.
 |
| Left to right: Haemoglobin, myoglobin, catalase, and ribonucletide reductase |
Therefore, whole process of Fe transport and storage keeps Fe under very close control; concentration of free iron in mammals is around 10-24 M.
In mammals, Fe from food is transported in blood by proteins called transferrins and it bounds Fe as shown below.
 |
| Binding pocket of transferrin |
In the Fe transport, Fe(III)/transferrin complex moves across lipid membrane by endocytosis.
 |
| Fe transport mechanism |
The key to controlling uptake and release Fe(III) is pH swing as phenolate and carboxylate bind Fe(III) strongly when deprotonated (pH 7.4) but not when protonated (pH 5.5).
Inside cell Fe is stored as ferritin until required, preventing undesired radical reactions. Ferritin consists of core of hydrated ferric oxide a protein overcoat. The protein overcoat is assembled from 24 protein units in roughly sphericla array.
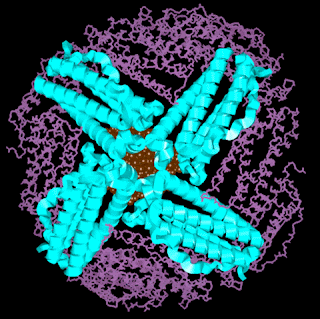 |
| Ferritin |
Inside core the iron crystallises with OH- ions and dihydrogen-phosphate ions to form a crystal with approximate stoichiometry is [FeIII9O9(OH)8(H2PO4)]. The structure is similar to that of mineral ferrihydrite, 5Fe2O3.9H2O, common in earth's crust but with dihydrogenphosphate terminating groups on exterior surface which anchor the protein overcoat to the ferric oxide core.
All the Fe in human body and mammals are taken from their diet such as from meat or Fe-rich plants. On the other hand, lower organisms such as bacteria need to extract it from environment where it exists predominantly as insoluble minerals.
Bacteria excretes siderophores to strip Fe(III) from minerals and form soluble complexes which are absorbed by organism. They are the strongest known binders of Fe(III) and it is based on tris-catecholate or tris-hydroxamate ligands which gives octahedral Fe(III) complex.
Thus, combining three bidentate chelating sites in one molecule gives even stronger binding such as in enterobactin that is used by bacteria such E.coli to scavenge iron from environment.
 |
| Enterobactin |
The increase in binding affinity is due to enterobactin is a hexadentate ligand instead of three bidentate ligands. Besides that, preorganisation of ligand minimises cost of conformational change and increases binding affinity.
On the other hand, tris-hydroxamate siderophores such as ferrichrome (from) yeast and desferrioxamine give the same effect as in enterobactin. Ferrichrome is a cyclic hexapeptide with three chelating arms pendant from cyclic core and partly preorganised octahedral cavity for Fe(III).
As mentioned earlier, those ligands bind very strongly to Fe(III) so it gives a challenge for the release mechanism making Fe ions can be used for metabolism. Unaided release would be too slow to be biologically relevant, so there are three possibilities which may all occur for different system.
- Decompostion of siderophore ligand. For example, enterobactin contains a triester ring which easily hydrolysable and can be broken down by organism.
- Protonation of ligand such as in transferrin.
- Reduction of Fe(III) to Fe(II)m which makes metal 'softer' and reduces its affinity for the hard binding site.




Comments