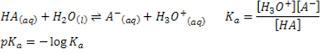Periodic Trends in Atomic Properties
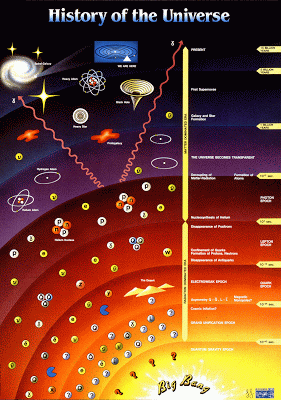
History of the universe In this section, we will try to see the trends in atomic properties of the periodic table, especially on s and p -block elements. The properties that wee will have a look are radius, ionisation energy, electron affinity, and electronegativity. Besides that, we will see how the electronegativity can predict the element characterisitic (metal, metalloid, and non-metal). Before we see its trends, we will have a quick journey at beginning of the universe. At the beginning of the universe, there were no elements or atomic nuclei; the atomic nuclei were formed around 1 minute after the big bang. Around 1 minute after the big bang, the universe had cooled sufficiently to form stable protons and neutrons. Then, protons and neutrons can fuse to give deuterium (H-2), isotope of hydrogen, nuclei. Then, deuterium nuclei fused to give stable He-4. After 3 minutes after the big bang, the universe is too cool for nuclear fusion to continue, so only light nuclei were for...