Acid and Base in Organic Chemistry
Acidity describes the tendency of a molecule to release a proton and can be quantified by acid dissociation constant (pKa). All molecules containing hydrogen have the potential to act as an acid and in chemistry the pKa value also has a reference point or reaction. In this case the reaction of acid with water.
Understanding of acidity trends in organic chemistry is very useful later on, especially to predict the mechanism reaction. From the equation above, we can simplify the if the acidity strength increases as value of pKa decreases.
Trends of pKa can be understood based on the stability of the anion that is formed on deprotonation. For atoms in the first row of the periodic table, the pKa decreases with increasing in electronegativity, because the negative charge on the anion is stabilised by the increased nuclear charge.
Moreover, going down a period, the pKa decreases, because the size of the atom increases, and the negative charge on the anion is spread out over a larger surface area. Hence, it provide less electron repulsion which give stability of anion.
Besides atomic radius and electronegativity, the hybridisation can also effect the acidity. The hybridisation state of the atom also has a significant effect on pKa, because it determines the average distance between the nucleus and the lone pair of electrons in the anion.
sp hybridisation has the most acidic proton compare the hybridisation, because sp hybridisation has the highest characteristic of s-orbital (50%) compare to the rest. This means, the negative charge on anion is closer to the nucleus, so it stabilise the negative charge.
Bonds between different elements are polarised due to differences in electronegativity. Consider the bonding in methanol, oxygen has a larger nuclear charge than carbon, therefore attracts the electron in the bond. These effects can be transmitted through single bond by inductive effect. Thus functional groups that stabilise negative charge (electron withdrawing functional groups) can act several bonds to reduce the pKa.
Besides that another factors can effect the value of pKa. Firstly, the distance of substituent:
or as the electronegativity of substituent increases, the acidity also increases.
Besides that, double bonds are more strongly polarised than single bonds, because both σ and π-electrons are attracted by the more electronegative element and inductive effects are correspondingly stronger.
Furthermore, alkyl substituent are weakly electron donating, but sp² and sp hybridised carbon centres are electron withdrawing.
Stabilisation of the negative charge on the anion by delocalisation has a much more dramatic effect on the pKa. This is caused the electrons are free to move through the entire conjugated π-system to find the lowest energy arrangement.
For example, the delocalisation of phenol and ethanoic acid conjugate.
a. Phenol conjugate. The charge is delocalised onto benzene ring.
b. Ethanoic acid. the charge is delocalised onto 2 oxygen atoms.
The delocalisation onto oxygen atom provides better effect of stabilisation because oxygen atom has higher nuclear charge than carbon.
The delocalisation effect is particularly important for deprotonation at carbon which is otherwise difficult.
e.g. deprotonation of pentane-2,4-dione.
The opposite of acidity is basicity, so basicity describes the tendency of a molecule to take up a proton and can be quantified by the pKa of the conjugate acid (the protonated form). To form the new bond to the proton, the molecule must use 2 electrons from a lone pair or π-bond. Hence, as pKa of the conjugate acid increases, the basicity increases.
pKa values can be used to predict the outcome of possible acid-base reactions. Significant proton transfer takes place if the base has a conjugate acid with a higher pKa than the acid. Another important consideration is the solvent; if the solvent has a lower pKa than the acid, it will be preferentially deprotonated.
Inductive effects also affect basicity in the same way as acid. As the electron withdrawing group is stronger, the conjugate acid is easier to release proton. Hence, the compound become less basic.
Besides that, hybridisation can affect the basicity. As the conjugate acid is sp hybridised, the positive charge is closer to the nucleus, so it destabilise the charge. Hence, it become less basic and the strongest basicity is at sp3.
Moreover, delocalisation has a dramatic effect on basicity. If the lone pair on the base is stabilised by delocalisation, the basicity is reduced. In the other sides, if the positive charge in the protonated form can be delocalised, the basicity is increased.
Understanding of acidity trends in organic chemistry is very useful later on, especially to predict the mechanism reaction. From the equation above, we can simplify the if the acidity strength increases as value of pKa decreases.
Trends of pKa can be understood based on the stability of the anion that is formed on deprotonation. For atoms in the first row of the periodic table, the pKa decreases with increasing in electronegativity, because the negative charge on the anion is stabilised by the increased nuclear charge.
Moreover, going down a period, the pKa decreases, because the size of the atom increases, and the negative charge on the anion is spread out over a larger surface area. Hence, it provide less electron repulsion which give stability of anion.
Besides atomic radius and electronegativity, the hybridisation can also effect the acidity. The hybridisation state of the atom also has a significant effect on pKa, because it determines the average distance between the nucleus and the lone pair of electrons in the anion.
sp hybridisation has the most acidic proton compare the hybridisation, because sp hybridisation has the highest characteristic of s-orbital (50%) compare to the rest. This means, the negative charge on anion is closer to the nucleus, so it stabilise the negative charge.
Bonds between different elements are polarised due to differences in electronegativity. Consider the bonding in methanol, oxygen has a larger nuclear charge than carbon, therefore attracts the electron in the bond. These effects can be transmitted through single bond by inductive effect. Thus functional groups that stabilise negative charge (electron withdrawing functional groups) can act several bonds to reduce the pKa.
Besides that another factors can effect the value of pKa. Firstly, the distance of substituent:
or as the electronegativity of substituent increases, the acidity also increases.
Besides that, double bonds are more strongly polarised than single bonds, because both σ and π-electrons are attracted by the more electronegative element and inductive effects are correspondingly stronger.
Furthermore, alkyl substituent are weakly electron donating, but sp² and sp hybridised carbon centres are electron withdrawing.
Stabilisation of the negative charge on the anion by delocalisation has a much more dramatic effect on the pKa. This is caused the electrons are free to move through the entire conjugated π-system to find the lowest energy arrangement.
For example, the delocalisation of phenol and ethanoic acid conjugate.
a. Phenol conjugate. The charge is delocalised onto benzene ring.
b. Ethanoic acid. the charge is delocalised onto 2 oxygen atoms.
The delocalisation onto oxygen atom provides better effect of stabilisation because oxygen atom has higher nuclear charge than carbon.
The delocalisation effect is particularly important for deprotonation at carbon which is otherwise difficult.
e.g. deprotonation of pentane-2,4-dione.
pKa values can be used to predict the outcome of possible acid-base reactions. Significant proton transfer takes place if the base has a conjugate acid with a higher pKa than the acid. Another important consideration is the solvent; if the solvent has a lower pKa than the acid, it will be preferentially deprotonated.
Inductive effects also affect basicity in the same way as acid. As the electron withdrawing group is stronger, the conjugate acid is easier to release proton. Hence, the compound become less basic.
Besides that, hybridisation can affect the basicity. As the conjugate acid is sp hybridised, the positive charge is closer to the nucleus, so it destabilise the charge. Hence, it become less basic and the strongest basicity is at sp3.
Moreover, delocalisation has a dramatic effect on basicity. If the lone pair on the base is stabilised by delocalisation, the basicity is reduced. In the other sides, if the positive charge in the protonated form can be delocalised, the basicity is increased.
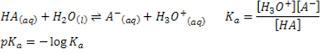













Comments