Organic Chemistry for A-Level: Carbonyls Chemistry and Nitrogen Compounds
A. Carbonyl Compounds (Aldehydes and Ketones)
The main feature of carbonyl compounds is there is at least one atom carbon has double bond with oxygen atom. There are two types of carbonyl compounds which are aldehyde and ketone. The structure of aldehyde and ketone are shown below.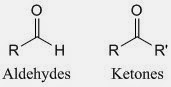
Aldehyde and ketone is a pair of functional group isomer as illustrated below.

The formation of carbonyl compounds involves oxidation reaction of alcohols.

The oxidation of secondary alcohol using acidic aqueous dichromate would give ketone as illustrated above. In the same way, we can think oxidising primary alcohols with the same reagent would give aldehyde. However, the oxidation of primary alcohols in aqueous solution would give carboxylic acids instead of aldehydes. The problem of this reaction is the presence of water that makes aldehyde is oxidised further so to overcome this problem the reaction should be done in water-free condition, i.e. in organic solvent. However, another problem is dichromate ions is insolube in most of organic solvent; hence modified oxidising agent which is soluble in organic solvent is required to form aldehydes such as pyridinium chlorochromate (PCC).

1. Nucleophilic addition
Carbonyl compounds could undergo addition reaction. However, it is different from alkene which undergoes electrophilic addition; aldehydes and ketones undergo nucleophilic addition. Nucleophile is defined as a chemical that can donate a pair of electron with the subsequent formation of a covalent bond. Basically we can identify a nucleophile by its negative charge or a pair of free electrons. Therefore, nucleophilic addition is addition reaction in which the first step is the attack by nucleophile on the electron-deficient part of molecule. The electron-deficient part is a part that has partially positive charge (δ+) and in carbonyl compound is shown on the dipole diagram below.
a. Reaction of carbonyl with cyanide.
The reaction between carbonyl and cyanide will produce the organic compound which has alcohol functional group and –CN functional group. For example, the reaction mechanism between propanal and cyanide is shown below. The reagent commonly is NaCN in HCN.
Could you suggest the product and the mechanism of this reaction below?

Would you expect A has optical isomers?
2. Identification reactions
a. Reaction with 2,4-DNPH (2,4-dinitrophenylhydrazine)This reaction can be used to identify a carbonyl compound. If in the sample there is a carbonyl compound, either aldehylde or ketone, it would give yellow precipitate of hydrazone. The reaction is shown below.

b. Fehling’s Reagent (an alkaline solution of Cu2+ ions complex)
Aldehydes: Red precipitate and carboxylic acid forms.
Ketones: No changing
c. Tollen’s Reagent (an aqueous solution of AgNO3 in excess ammonia)
Aldehydes: Silver mirror and carboxylic acid forms.
Ketones: No changing
d. Acidified Cr2O72-
Aldehydes: The solution turns green (Cr3+)
Ketones: No changing (solution still yellow-orange)
e. An alkaline solution of iodine
This test is basically to identify CH3CO– functional group and if the result is positive it would give yellow precipitate of triiodomethane.

The reaction proceeds via

B. Carboxylic acids and its derivates
Carboxylic acid has certain main features. Firstly, it has the acidic properties and carboxylic acid is commonly known as the strongest acid in organic compound. Therefore, carboxylic acid could also react in similar way as acid-base reaction as shown on the reaction below.
Secondly, carboxylic acid also has relatively higher boiling point along in organic compound even compare with alcohol. This is caused by carboxylic acid has stronger and more hydrogen bonding compare with alcohol.

The synthesis of carboxylic acid could be done in many ways, and the simplest one is oxidation of primary alcohol or aldehyde. However, carboxylic acids cannot be reduced by NaBH4 or LiAlH4 in dry ether because its acidic nature. The proton will simply react with the hydride ion to produce H2 gas.

Another way to synthesis carboxylic acid is from nitriles hydrolysis.

The reaction proceeds via formation of amide as an intermediate followed by its hydrolysis

1. The acidity of carboxylic acids
Since carboxylic acids are one of the types of acid, it might be useful to know its acidity. Acidity in the simplest definition is the easiness of a compound to release H+ (proton) and can be analysed quantitatively using Ka or pKa (pKa = -log[Ka]). If pKa is higher, it means the acid becomes weaker or more difficult to release proton. Carboxylic acids in water release proton as show in reaction below.
The dashed line on the CH3COO- means there is a resonance structure due to electron delocalisation. The delocalised electron system is formed because of p orbital in the COO system to form π bond.

When a carboxylic acid has electron-withdrawing functional group such as Cl atom, it becomes a better acid than the unsubstituted carboxylic acid.

As we see in the series above, when ethanoic acid has more Cl atoms on C2, it becomes stronger acid. This is an example of substituent effect. The greater acidity of chloroethanoic acid can be attributed, in part, to the extra electron-attracting inductive effect of the electronegative chlorine atom. By adding its inductive effect to that carbonyl group and the oxygen, it makes hydroxyl proton of chloroethanoic acid even more positive than ethanoic acid. It also stabilise the chloroethanoate ion that is formed when the proton is lost by dispersing its negative charge.

Since inductive effect are not transmitted very effectively through covalent bonds, the acid strengthening effect decreases as the distance between the electron-withdrawing group and the carboxyl group increases.
2. The reactions
Besides it can undergo acid-base reaction, there are many types of organic reaction involving carboxylic acids. In this part, it will focus on esterification, synthesis of acyl chloride, amide.a. Synthesis of acyl chloride
Carboxylic acid could form acyl chloride by reacting carboxylic acid with SOCl2 or PCl3 or PCl5.
Acyl chloride is commonly used as a precursor compound for certain organic synthesis such as amide synthesis.
b. Esterification
Esterification basically is a synthesis of ester. Ester has a main characteristic fruity smells and basically could be formed by reacting carboxylic acid or acyl chloride with alcohols with helps acid catalyst.
The reaction proceeds via mechanism below.

Acyl chloride is more reactive than carboxylic acid and this is can be really helpful for ester phenol synthesis.

Moreover, the reaction between dicarboxylic acids and diols can form polymer and the polymer is called polymer condensation due to the formation of small condesate or water.

c. Amide formation
Amide can be made by reacting acyl chloride with amine but this reaction would not work using carboxylic acid. Why an amide would not form from the reaction between carboxylic acid and amine?
The reaction proceeds via:

This functional group can also form a polymer by reacting diamine with diacyl chloride such as the formation of nylon 6,6.

If all compounds above (esters, acyl chloride, and amides) react with water with help either acid catalyst or base catalyst, it will give carboxylic acid as one of the products. Therefore, those molecules are called carboxylic acid derivates. The diagram below show how all those compounds are related to each others.

As note from this diagram, hydrolysis carboxylic acid derivates under basic condition would produce carboxylate ions rather than carboxylic acid.
C. Nitrogen Compounds
Nitrogen compounds are commonly known as amine compounds and it is basically the ammonia derivates. Therefore, the main features of amines are similar with ammonia. Amines have relatively high boiling point compare with the other organic compounds but it is still lower than alcohols. Besides that, due to its polarity shorter chain of amines has higher solubility in water and polar solvent. Furthermore, amine has alkaline properties. Amine also can be classified as shown below.
Furthermore, amine also exist in nature especially in alkaloid compounds and amino acids.

Amine can be synthesised in many way. Firstly, by substitution reaction of alkyl halide with ammonia.

Secondly, amine could be formed from reduction of nitrile compounds

Besides that, alkylamine could act as nucleophile in substitution reaction due to the lone pair electrons on N atoms.

Alkylation of amine as shown above could lead to a mixture of several amine compound which makes synthesising secondary amine is quite tricky using this pathway. Furthermore, the yield of higher amine compound in that reaction is getting lower from primary to quaternary due to steric hindrance from ethyl group. To overcome this "messy" reaction, secondary amine can be synthesised using carbonyl compounds via formation of imine followed by reduction.

Comments