Organic Chemistry for A-Level: Benzene and Its Derivates
A. Structure
The structure of benzene was suggested firstly by Kekulé as a cyclic molecule but he also thought benzene had alternative single and double carbon-carbon bonds.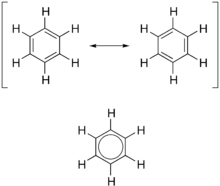
The Kekulé structure is expected that benzene undergoes addition reaction. However, benzene is far less easily to undergo addition reaction than alkene, such as cyclohexene. Besides that, the carbon-carbon bond lengths of benzene are all identical, with the lengths intermediate between those single and double bonds.
The current model of benzene accounts for these observations. Each carbon contributes one electron to a π bond. However, the pi bonds formed do not lie between pairs of carbon atoms as in an alkene: the π bonds spread over all six carbon atoms. The electrons occupy three delocalised π orbitals. The π molecular orbitals are formed from the overlapping of carbon p atomic orbitals. To achieve maximum overlap, the benzene molecule must be planar and as well the hybridisation of carbon atoms in benzene is all sp2 which gives planar structure.

B. The reactions
Since benzene has delocalised electrons, so it is difficult for benzene to undergo addition reaction. Benzene can undergo substitution reaction, instead of addition reaction. Because benzene has high density of electrons, so it undergoes electrophilic substitution.1. Halogenation (e.g. chlorination)
The substitution of H on benzene with halogen is done with helps of Lewis acid catalyst such as AlCl3 or FeBr3.
The reaction proceeds via:

In this reaction, chlorine combines with AlCl3 to form a complex. Then, the benzene ring donates an electron to the terminal chlorine, forming the arenium ion and neutralising the formal positive charge on the chlorine. A proton is removed from the arenium ion to form chlorobenzene and regenerate the catalyst.
2. Nitration

The reaction proceeds via:

The first step is the protonation of HNO3 to form a nitronium ion. The nitronium ion is the electrophile in nitration; it reacts with benzene to form a resonance-stabilised arenium ion. The arenium ion then loses a proton to a lewis base and becomes nitrobenzene.
3. Sulphonation

The reaction proceeds via:
In this reaction, HSO3+ is the electrophile that reacts with benzene to form an arenium ion. A proton is removed from the arenium ion to form benzenesulfonate ion. The benzenesulfonate ion accepts a proton to become benzenesulfonic acid.
4. Friedel-Crafts Alkylation
The reaction proceeds via:The acid-base lewis reaction forms a complex and the complex dissociates to form a carbocation. The carbocation acts as electrophile, reacts with benzene to form arenium ion. A proton is removed from the arenium ion to form toluene. This step regenerates the AlCl3 and liberates HCl. However, this reaction has some drawbacks for putting longer alkyl chain. Firstly, further substitution can occur due to electron-donating nature of alkyl group. Secondly, this reaction can work efficiently until ethyl group because longer alkyl chain would form a primary carbocation which can undergo rearrangement to form stable carbocation.

5. Friedel-Crafts Acylation

This reaction proceeds via:

Acyl chloride undergoes lewis acid-base reaction to form a complex and the complex can dissociate to form acylium ion. The acylium ion acts as an electrophile and it reacts with benzene to form arenium ion. Then, a proton is removed from the arenium ion, forming aryl ketone.
This acylation can be used to overcome the problems of Friedel-Crafts alkylation by forming an acylbenzene followed by reduction.

This reaction works efficiently because the nature of electron-withdrawing of acyl group which makes the benzene ring less reactive; hence further substitution would not occur.
6. Synthesis of Benzoic acid (Oxidation reaction)
Strong oxidising agents would oxidise toluene to benzoic acid. The oxidation can be carried out by the action of hot alkaline potassium permanganate.
An important characteristic of side-chain oxidations is that oxidation takes place initially at the benzylic carbon. Alkylbenzenes with alkyl groups longer than methyl are ultimately degraded to benzoic acids. Side-chain oxidation is not restricted to alkyl groups. Alkenyl, alkynyl, and acyl groups are also oxidised by hot alkaline potassium permanganate.
7. Disubstitution of benzene
In benzene reaction, it is possible to get further substitution. In the second substitution, there are 3 possible positions of the electrophile to attack:
The position of second functional group depends on the first functional group. Therefore, there are two types: ortho-para directing groups and meta directing groups.
a. Ortho-para directing groups
Ortho-para directing groups are mainly activating groups. The activating groups make the benzene ring is more reactive or the ring is activated.
From the scheme above, it shows the reason why the electrophile prefers ortho (1,2) or para position (1,4) in the presence of electron-donating group. The arenium ion intermediate is stabilised further in ortho and para position as the positive charge can be at 1-position (ipso) where the electron-donating group attached while 1,3 position does not have this intermediate.
The general feature of the activating groups is the functional group has lone pair electron such as -OH, -NH2. Moreover, alkyl group is also the activating group via inductive effect.

In the other sides, halogens (Cl or Br) are ortho-para directing groups despite the halogens is electron-withdrawing group due to its electronegativity. Halogens can donate the electron via resonance structure which has stronger effect than the electron-withdrawing effect via inductive effect.
b. Meta directing groups
Meta directing groups are the opposite of ortho-para directing groups, so meta directing groups are deactivating groups. The deactivating group withdraws the electrons from the ring which makes it electron poor and it reacts slower with an electrophile. Therefore, the general feature of the deactivating group is the functional group has partially positive charge. For example, -COOH is one of the deactivating group.
The preference of meta position is due to the charge positive would no be distributed to ipso position in meta position as shown below.

In ortho and para substitution, the positive charge is distributed also to the ipso position which means it is connected to the electron withdrawing. This position would destabilise the intermediate.
To sum up, the common functional groups can be classified as:

C. Phenylamine
1. The basicity of phenylamine
Phenylamine is much weaker bases than alkylamines. The base strengths of phenylamine could be compared with methylamine with relative to ammonia.
The order of base strength is due to the inductive effects of the methyl and phenyl groups.
Alkyl groups have a positive inductive effect. This means that they have a tendency to push electrons towards a neighbouring atom. In methylamine, the effect of this is to increase slightly the electron density on the nitrogen atom. This increased charge density on the nitrogen atom enhances its ability to donate its lone-pair electrons to a proton, so methylamine is a stronger base than ammonia.
The phenyl group has negative inductive effect. The electron charge density on the nitrogen atom in phenylamine is decreased. Consequently, the ability of phenylamine to accept a proton is decreased, so it is a weaker base than ammonia. This effect is further enhanced in phenylamine because the lone pair of electrons on the nitrogen atom becomes partially delocalised over the benzene ring.
2. The reactions
a. Reaction as alkaline
Since phenylamine has alkaline properties, so phenylamine reacts with acid to form salt (just acid-base reaction).b. Synthesis arylamide
Phenylamine can form an amide with acyl chloride.
Arylamide is one of the precursor compounds for synthesis secondary phenylamine. Furthermore, it could be transformed into tertiary phenylamine.

c. Formation diazonium and its coupling reaction
Diazonium salt is a good precursor for many organic syntheses. Diazonium salts is formed from amine or phenylamine reacts with NaNO2, HCl at low temperature.
Diazonium salts is commonly used to synthesis reaction for:
- Halogenation
- Formation of phenol
- Deamination
- Coupling reaction of arenediazonium salts

Arenediazonium ions are weak electrophiles; they react with highly reactive aromatic compounds – with phenols or tertiary arylamines – to yield azo compounds. This electrophilic aromatic substitution is often called a diazo coupling reaction.

Coupling between arenediazonium and phenols take place most rapidly in slightly alkaline solution. In the other sides, the reaction with tertiary arylamines takes place most rapidly in slightly acidic solution. This azo compounds are quite common as a colouring agent for fabric as well as for food.

D. Phenol
1. The acidity of phenol
Phenol ionises slightly in water. The O-H bond in phenol breaks to form a hydrogen ion and negative phenoxide ion. This bond breaking occurs more readily in a phenol molecule than in a water molecule because the phenoxide ion is stabilised by a partial delocalisation over the benzene ring of the negative charge on the oxygen atom. Phenol is therefore more acidic than water.
Ethanol ionises even less than water. The positive inductive effect in ethanol increases the electron charge density on the oxygen atom. This increases the ability of the ethoxide ion to attract hydrogen ions, so ethanol is less acidic than water.

When phenol has another functional group, the acidity will change as well. When there is electron-withdrawing group in phenol, phenol becomes stronger acid. It is caused by the electron-withdrawing group spreads out the anionic charge of phenoxide, in the other words the delocalised electrons of phenoxide ion is stabilised. In the other sides, electron-donating group would destabilise the phenoxide ion.
2. The reactions
a. The formation of phenol
Mainly phenol could be formed from reaction diazonium salts with water (see the reaction of diazonium above).b. Phenol identification
Phenol could be detected in sample by reaction with Br2 in CH3COOH.
Decolouration of bromine colour is expected in the presence of phenol.
c. Esterification
Phenol could be a source of –OH functional group the same way as alcohols. However, ester cannot be formed from the reaction of phenol and carboxylic acid. To form phenol ester, phenol should react with carboxylic derivates which are more reactive such as acyl chloride or anhydride carboxylic acid.
d. How to distinguish with alcohol?
Both phenol and alcohol could react with sodium metal
However, alcohol cannot react with NaOH but phenol can react with NaOH.




Comments