In the recent decade, chemists have taken nature as its inspiration in many ways; one of them is in medicinal chemistry. Many natural products have been screened for their bioactivities to develop new generation of therapeutic agents for 'incurable' diseases such as cancer. However, there is one major drawback from taking natural product as drug. This problem is its complex structure which makes it difficult to synthesise especially in large scale.
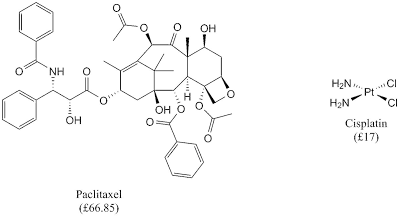 |
| Anticancer drugs: natural products (paclitaxel) and coordination complex (cisplatin) |
Luckily, a simple coordination complex, such as cisplatin, gives similar efficacy as natural product-based drug and simple coordination complex is used to treat 80% of all solid cancers. It is noteworthy that the market for this drug is around $1 billion.
Cisplatin or
cis-DDP was first reported in 1845 and in 1893 Alfred Werner deduced its structure and described its
cis- and
trans- isomers. However, the story of Pt-based drug cancer begun much later in 1962 when physicist Barney Rosenberg was intrigued by the similarity in structures produced in cell mitosis and the field lines that result when poles of opposite electromagnetic polarity are brought close together.
Then, he collaborated with biologists to grow cells in the presence of a fluctuating electromagnetic field. His used prokaryotes E. coli as a test case in a medium enriched with glucose and magnesium chloride and current oscillation of 50 - 100 000 cycles s
-1 was applied to the growing medium using Pt electrodes. Interestingly, the application of current stopped cell division but not cell growth and these was observed in the presence of oxygen. Rosenberg and his colleagues deduced that it was not due to electric field oscillation, but was caused by a new chemical species that was an oxidising agent.
 |
| Formation of Pt(IV) salts in growing medium |
Then, it was established that Pt(IV) salts could be obtained via electrolytic processes and in the presence of light this could further react to form neutral diamine species with the
cis- form is the only active species
From this research, several Pt(II) and Pt(IV) were screened and it was shown only
cis- forms were the active species with
cis-Pt(NH
3)
2Cl
2 as the most active species. The clinical trials of
cis-Pt(NH
3)
2Cl
2 began in 1972 and it was launch worlwide in 1979 and rapidly became the most widely used anticancer drug in USA, Europe, and Japan.
The efficacy of cisplatin raised several questions regarding its mode of action. It was known that several agents that inhibit cell division but not growth, such as UV radiation, are known to inhibit DNA synthesis. When cells are treated with one therapeutic, it preferentially inhibits DNA synthesis over RNA and protein synthesis. This evidence showed a direct interaction with DNA and DNA polymerase are not affected by this treatment. Furthermore, cisplatin non-competitively inhibits the binding of the intercalator ethidium bromide; thus, Pt complex covalently bound to DNA.
The binding mode of cisplatin with DNA is closely related with cisplatin chemistry in aqueous environment where the extent of dissociation is dependent on the ambient chloride concentration.
 |
| Aqueous chemistry of cisplatin |
In blood plasma, chloride ions are in high concentration which would push the equilibrium in favour of neutral lipophilic cisplatin. However, when cisplatin is in the cell, hydrolysis products are favoured due to low concentration of chloride ions. This products would lead to cisplatin-DNA adducts.
The next question would be where cisplatin binds to DNA and it can be figured out by surveying the possible binding site on the DNA bases.
 |
| Binding site for Pt adducts |
There are several possibilities of the binding sites for Pt from the bases, N1 of adenosine, N7 of adenosisne and guanidine, and N3 of cytosine. However, N1 of adenosine and N3 of cytosine are less availabe in DNA to hydrogen bonding interaction. Then, N7 of guanidine is a stronger lewis base than N7 in adenosine. Hence, N7 of guanidine is the preferred binding site for Pt adducts and these are several possible structure of Pt-DNA adducts.
 |
| The possible structure of DNA-Pt adducts |
When Pt forms intrastrand adducts, the guanosine base planes are completely destacked which disrupt the DNA sequence. At this structure, the bases are now held at dihedral angel of 76-87° and it produce a large bend in duplex structure. This distortion changes the sugar conformation into more rigid structure and it restricts the conformational flexibility of the DNA backbone. The distorted DNA can help to rationalise how cisplatin works.
In recent research, it has revealed that both transplatin and cisplatin affect DNA replication; yet transplatin is still clinically inactive. Although inhibition of DNA replication can explain some of the biological effects of cisplatin, it does not explain its anticancer properties. In some experiments, cisplatin inhibited transcription process, while transplatin shows no effect. This suggests that anticancer properties of cisplatin are due to transcription inhibition.
There are several mechanisms have been considered for how trancription could be blocked. In the late 1980s/early 1990s, it was established that high mobility group (HMG) domain proteins bind to cisplatin modified DNA. The HMG domain is a DNA-binding motif found in a large number of proteins, including transcription factors. They bind in the minor groove and bend DNA on binding. Subsequently, it was confirmend that the HMG domain binds specifically to 1,2-
cis-GG and 1,2-
cis-GA adducts.
 |
| Structure of a HMG domain bound to a platinated oligonucleotide (left) and a schematic diagram of the hijacking and repair shielding hypotheses. |
From this idea, there are two of the more prominent hypotheses have been explored. First hypothesis is that cisplatin-DNA adducts hijack proteins away from their normal binding sites, thus disrupting cellular function. Since many HMG-domain proteins function as transcription factors, their removal from promotor or suppresor sequences by binding to cisplatin-DNA adducts could severely alter tumour cell biology. The other hypothesis suggests that HMG-domain proteins could block cisplatin-DNA adducts from damage recognition needed for repair. This activity would result in diminished repair of the adducts, and the persistence of Pt on the DNA could lead to cell death. Hence, this hypothesis is referred as repair shielding hypothesis. It is worthy to note that these two models are not mutually exclusive and could work in concert to effect cisplatin cytotoxicity.
In short, we have seen how cisplatin set a new generation of anticancer agent which based on a humble coordination Pt complex. This field is still an emerging field as there are several issues regarding cisplatin, such as it can only be administered intravenously and low aqueous solubility, that needs to be addressed. Hence, the discovery of new generation of Pt drugs is still ongoing..
 |
| New generation of Pt drugs |
Reference
E. R. Jamieson and S. J. Lippard, Chem. Rev., 1999, 99, 2467 - 2498







Comments