The Most Noble Complex Compound
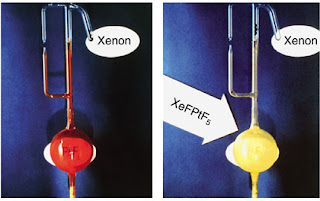 |
| Bartlett's oxidation of Xe using PtF6 |
The original study of this synthesis was the preparation of the mysterious gold(I) fluoride, AuF, via reduction of AuF3 in the presence of weakly coordinating agent. The initial experiment was using AsF3 in HF/SbF5 as reducing agent and it afforded the first derivative of AuF. Then, by replacing with milder reducing agent Xe, the reduction stopped at Au2+ and resulted the unexpected dark red crystal [AuXe4]2+ that can be grown at -78 °C. Removal of Xe under vacuum results in the known Au(SbF6)2. Then, addition of Xe to Au2+ in HF/SbF5 gives a dark red solution at -40 °C. It is noteworthy that the reaction between Xe and Au2+ is reversible and it requires a xenon pressure of 10 bar to stabilise [AuXe4]2+.
 |
| The crystal structure of the cation [AuXe4]2+ in [AuXe4][Sb2F11]2. |
Interestingly, in this complex Xe acts as σ donor towards Au2+ and this is reflected in the calculated charge distribution where the main part of the positive charge resides on the Xe atoms. This large charge transfer may be explain due to strong relativistic effect on gold atom. Hence, the bonding between xenon and gold in this compound may not be different from that between xenon and any electronegative main-group elements.
During the reduction and the complexation, the presence of extreme Brønsted acid HF/SbF5 is essential. The Au3+ ion in fluoride systems is normanyl as [AuF4]-, which then protonated to form Au(HF)4, which can be considered as naked Au3+; hence, it has a much higher oxidation potential.
[Au(HF)n]2+ + 4Xe ⇌ [AuXe4]2+ + nHFThen, the complexation reaction must be an equilibrium, which again can only proceed if Xe is the strongest base in the system.
AuF3 + 6Xe + 3H+ → [AuXe4]2+ + [Xe2]+ + 3HFFurthermore, in the overall reaction indicates again the role protons and the formation of green [Xe2][Sb4F21] crystals, which was detected in the solid reaction mixture at -60 °C.
This study demonstrated a novel Xe compound where it is bonded to a metal but the isolation of [AuXe4]2+ raises many questions such as whether this compound remains unique or if this is the first of a series of a new complexes. Besides that, it is difficult to predict the nature of this complex since it is unique in another way: stoichiometry and structure of Au2+ complex are rare if not entirely new.
Reference
S. Seidel and K. Seppelt, Science, 2000, 290, 117-118.
Comments