It was isolated for the first time in 1966 from the plant
Rhazya stricta,
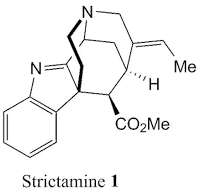
strictamine (1) has been a great interest due to its unique properties. Strictamine belongs to the group of akuammiline alkaloids which is the family of monoterpenoid indole alkaloids.
This compound has been showed to inhibit the nuclear factor-𝜅B (NF-𝜅B), which plays an important role in immune and inflammatory responses. Apart from its bioactivity, strictamine has attracted synthetic chemists because its structure bearing a tricyclic unit of methanoquinolizidine. This interesting challenge causes only four groups - Garg (enantioselective), Zhu (racemic), Ohno (formal synthesis), and Gaich (formal synthesis) - have managed to complete a total synthesis of strictamine. This time on "
Totally Synthesised", we will see synthetic strategy by Gaich's group which used [2,3]-Stevens rearrangement to build the methanoquinolizidine unit.
 |
| Cage-like methanoquinolizidine 2 of the akuammiline alkaloids. |
The structure of strictamine is characterised by its methanoquinolizidine unit (highlighted in red) which represent a tricyclic system. This unit is assembled by C7-C16 (biosynthetic numbering) and this bond is crucial to break the aromatic system of indolenine system (highlighted in blue) due to quaternary carbon at C7. This unique structure leads to a compact, cage like structure which is comparable to adamantane.
 |
| Establishment of quaternary carbon centre in 5 |
The synthetic strategy by Gaich's group started with α-cyanoacetate, which underwent alkylation with dibromoethane to construct the quaternary C7 centre. Then, lactam formation was done by sodium hexamethyldisilazane (NaHMDS) followed by
tBoc protection. The protected lactam was reduced to give hemiaminal
6 where the OH group would be substituted by allylic group via OTs group. The allylic group is important in this tranformation where it underwent isomerisation into
E-propenyl by 2nd generation Hoveyda-Grubbs catalyst followed by reduction of ethyl ester and protection by MOM group to give
8. The
E-propenyl group was ozonolysed followed by Pinnick oxidation to give
9.
 |
| Establishment of the α-stereocentre in 12 |
Then,
9 was deprotected using TFA and
N-allylic group was introduced using allylic bromide to give
10. Then, introduction of the tertiary α-stereocentre at the pyrrolidine ring was done via Stevens rearrangement, intermediate
11, to give
12. Interestingly, this process was diastereoselective (99:1) and it might be due the steric hindrance from nitrophenyl group. Therefore, the α-stereocentre is
cis towards the MOM group. Then,
N-allylic group was removed under Pd-mediated reaction and reprotected by Fmoc group. Then, ozonolysis of allylic group at α-stereocentre afforded the alcohol which then transformed into vinyl group via trifilation, S
N2-reaction with NaSePh, and NaIO
4 oxidative workup to give
15.
 |
| Final steps of the synthesis of strictamine 1 |
The deprotection of MOM group in
15 followed by Dess-Martin periodinane (DMP) gave aldehyde group in
16 which then oxidised using Pinnick oxidation and transformed into acyl chloride using Ghosez reagent to give
17. This acyl chloride group was then transformed into diazoketone
18 by reacting with diazomethane in sealed container. The formed
18 was then deprotected and subsequently triggered the formation of bridged bicyclic
19 which is important in this synthesis. The bridged bicyclic system acts as a precursor for another Stevens rearrangement to give
22. This compound now has the correct structure, 2-azabicyclo[3.3.1]nonane system (highlighted in red), leading to methanoquinolizidine unit in
1. The indolenine unit was formed under reduced condition but it formed partially reduced nitrone
23 instead of indolenine unit. Hence, to form the correct oxidation level of indolenine, an extra step was needed where
23 was reacted with PBr
3 and the synthesis was finished off using Ni(0)-mediated ring closing reaction.
In this synthetic strategy, Gaich's group shows the versatility of a [2,3] Stevens rearrangement for the construction 2-azabicyclo[3.3.1]nonane system. Besides that, this synthetic route is highly diastereoselective, very robust, and can be performed in multigram scale,
Reference
R. Eckermann, M. Breunig, and T. Gaich,
Chem. Commun., 2016,
52, 11363-11365.




Comments