Powering the Molecular World
In biological process, molecular machines are one of the examples of the most intricate functional molecules and have been attracted many chemists recently. Inspired from this idea, synthetic molecular machines have been developed ranging from synthesizers to motors, but there are no synthetic molecule machines that operate autonomously using chemical energy; in biological process ATP is used as the chemical energy or fuel.
Leigh's group from the University of Manchester successfully created a system in which small molecular ring is transported around a cyclic molecular track when powered by irreversible reaction.
Leigh's autonomous molecular motor is based on [2] catenane system featuring two interlocked molecular rings of different sizes where fumaramide residues on the larger track serve as binding site for smaller benzylic amide macrocycle. The presence of bulky 9-fluorenylmethoxycarbonyl (Fmoc) groups block the passage of the small ring and when both groups are attached, it traps the small ring in one or other fumaramide sites. Furthermore, one of the fumaramide sites is deuterated to help monitoring the position of small ring using 1H-NMR.
When one of the bulky groups are cleaved via chemical reaction, it allows the small ring to move back and forth between the two fumaramide sites using the unblocked pathway.
Starting from 2 and 2', Fmoc attachment, i.e. carbonate formation, prefers where the small ring at distant, so attaching Fmoc to 2 should preferentially form the positional isomer of FumD2-1 and likewise attaching Fmoc to 2' should favour FumH2-1. This implies each reaction causing a net clockwise direction. The cleavage of either of the Fmoc groups then occurs (the kinetic of the cleavage is not affected by the position of the small ring); hence 2 and 2' are formed in equal amounts and this allows another Fmoc attachment to occur with net directional movement of the small ring. Furthemore, to maximise the efficiency of the process, sufficient amount of Fmoc-Cl fuel needs to be present promoting the attachment process whenever one of the hydroxyl groups is unmasked. This also prevents both hydroxyl groups unmasked which causing the undirectional biased movement of the small ring. Furthermore, chirality does not affect direction of the rotation of this system.
It is noteworthy that this system operates autonomously as long as unspent Fmoc-Cl fuel is present and during the motor operating condition both attachment and cleavage reactions occur.
Comparing this molecular motor with the motor protein, both of them have similarity in certain aspects, such as they serve as catalyst. In motor protein, it serve as catalyst for ATP hydrolysis while in this system it catalyse the conversion of Fmoc-Cl and Et3N into dibenzofulvene, CO2, and Et3NHCl. For both systems, the free energy released drives the directional displacement of the motor components. In the other words, the consumption of two molecules of Fmoc-Cl is required to power benzylic amide macrocycle lapping full rotation around the catenane track.
Reference
M. R. Wilson, J. Solà, A. Carlone, S. M. Goldup, N. Lebrasseur, and D. A. Leigh, Nature, 2016, 534, 235-240.
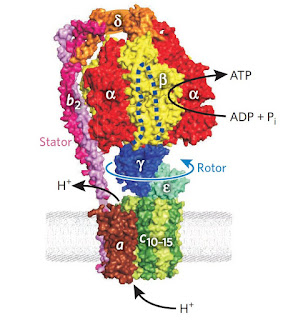 |
| One of the example of motor protein: ATP synthase. (J. Weber, Nat. Chem. Biol., 2010, 6 794-795) |
 |
| The structure of catenane rotary motor 1 |
When one of the bulky groups are cleaved via chemical reaction, it allows the small ring to move back and forth between the two fumaramide sites using the unblocked pathway.
 |
| The operation of chemically fuelled [2] catenane rotary motor. |
It is noteworthy that this system operates autonomously as long as unspent Fmoc-Cl fuel is present and during the motor operating condition both attachment and cleavage reactions occur.
Comparing this molecular motor with the motor protein, both of them have similarity in certain aspects, such as they serve as catalyst. In motor protein, it serve as catalyst for ATP hydrolysis while in this system it catalyse the conversion of Fmoc-Cl and Et3N into dibenzofulvene, CO2, and Et3NHCl. For both systems, the free energy released drives the directional displacement of the motor components. In the other words, the consumption of two molecules of Fmoc-Cl is required to power benzylic amide macrocycle lapping full rotation around the catenane track.
Reference
M. R. Wilson, J. Solà, A. Carlone, S. M. Goldup, N. Lebrasseur, and D. A. Leigh, Nature, 2016, 534, 235-240.
Comments