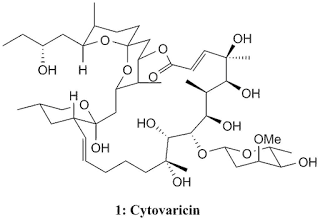
Cytovaricin
Cytovaricin, 1, was isolated by Isono's group in 1981 from a culture of Streptomyces diastatochromogenes and the structure was elucidated in 1983 from X-ray crystallography. From its structure, cytovaricin was revealed to have such an elegant and complex structure with 22-membered macrolide structure as its framework and possessing 17 stereocentres, a spiroketal, and a glycoside unit giving four more stereocentres. This highly complex molecule give a unique challenge to organic chemists in asymmetric synthesis.
The synthesis of cytovaricin by Evans and coworkers shows an elegant route to synthesise this highly complex molecule.
The structure elucidation of cytovaricin was triggered from the finding that it is a potent inhibitor against sarcoma cells in tissue culture which makes cytovaricin a new potential as antineoplastic antibiotic. Besides that, cytovaricin appears to be related biogenetically to the oligomycin/rutamycin antibiotic family.
The synthesis of cytovaricin by Evans and coworkers shows an elegant route to synthesise this highly complex molecule.
 |
| Retrosynthesis analysis of cytovaricin |
Its synthesis can be seen as joining two fragments, spiroketal unit 2 and polyol unit 3, using Julia olefination and macrolactonisation. In the synthesis of both fragments, the Evans asymmetric aldol reaction is featured heavily and it was achieved by using chiral auxiliary as shown below.
 |
| Evans asymmetric aldol and alkylation reaction |
The auxiliary oxazolidone unit directs the addition toward the carbonyl unit by providing a steric hindrance at one face of the carbonyl. Furthermore, interesting point to note is in using chiral auxiliary it is important that the auxiliary unit can be removed allowing to do further reactions.
The synthesis of the spiroketal unit was done in 10 steps with the Evans asymmetric aldol reaction was used twice to synthesis 6 and 17.
The first step of the synthesis was the Evans aldol reaction and after removal of the auxiliary the formed carboxylic acid is reduced and protected using triisopropylsilyl (TIPS) unit. Besides that, a unique amide moiety such as in 9 and 18 was used to form the carbonyl functional groups. In 9, this amide functional group was used in the formation of hydrazone 10 while in 18 it was reduced to form aldehyde which then used in Julia olefination with 3. The formation of spiroketal unit 13 from 12 was done by deprotection of triethylsilyl (TES) unit and the 1,3-diol that is formed from the iodohydration of 6. This spiroketal unit gives the main fragment of 2 which would be joined with the polyol unit of 3. In 2, different alcohol protecting groups were used and it gives the selectivity on deprotection which is important in the next step.
 |
| Formation of spiroketal unit 2 |
The second fragment of cytovaricin is the polyol unit 3 and it was synthesised in 8 steps and the Evans aldol was used twice in the synthesis of 22 and 24.
 |
| Formation of polyol unit 3 |
It was started from the same staring materal in spiroketal synthesis and in this step after aldol formation, the unique amide moiety was formed to undergo lithiation giving 22. Different asymmetric synthesis, apart from Evans aldol and alkylation reactions, was also used such as in the formation of 23 where the chiral alcohol directs the reduction to give syn-1,3-diol which then protected by tBu2Si unit. Interestingly, Evans asymmetric aldol reaction of 23 and 24 gives an anti-selectivity; it commonly gives syn-selectivity. To give the correct configuration, an inversion of stereocentre was done by using L-selectride. This inverted chiral centre was then connected to 27 to give the glycoside unit. The final steps of the synthesis of 3 were the formation of sulphonate groups which was done using Grignard reagent followed by oxidation by OsO4 and NMO, which also oxidised the alkene to give 1,2-diol, to give the sulphonate group. This functional group is then used for Julia olefination. On the other hand, the carboxylic acid was formed from Horner-Wandsworth-Emmons reaction using phosphonate 31 with the oxidised 30. This carboxylic acid is used in macrolactonisation (esterification).
The final steps of the synthesis were joining both fragments and deprotection reactions.
The first reaction for joining both fragments was the Julia olefination and then followed by estefication which preceded by the removal of CH2OCH2CCl3 protecting group by 4-DMAP.HCl. The esterication was done using DCC (N,N'-dicyclohexyldicarbodiimide) coupling agent. The final step of this synthesis to give cytovaricin was deprotection of the remaining protecting groups; DDQ removes PMB protecting groups and all the silyl protecting groups are removed by HF.py.
The synthesis of cytovaricin demonstrates the elegant uses of asymmetric synthesis to give the complex structure with many stereocentres in it. The use of Evans asymmetric aldol and alkylation reactions in this synthesis opens up a new way in polyketide synthesis with controlled stereochemistry of the product.
References
The final steps of the synthesis were joining both fragments and deprotection reactions.
The first reaction for joining both fragments was the Julia olefination and then followed by estefication which preceded by the removal of CH2OCH2CCl3 protecting group by 4-DMAP.HCl. The esterication was done using DCC (N,N'-dicyclohexyldicarbodiimide) coupling agent. The final step of this synthesis to give cytovaricin was deprotection of the remaining protecting groups; DDQ removes PMB protecting groups and all the silyl protecting groups are removed by HF.py.
The synthesis of cytovaricin demonstrates the elegant uses of asymmetric synthesis to give the complex structure with many stereocentres in it. The use of Evans asymmetric aldol and alkylation reactions in this synthesis opens up a new way in polyketide synthesis with controlled stereochemistry of the product.
References
- D. A. Evans, S. W. Kaldor, T. K. Jones, J. Clardy, and T. J. Stout, J. Am. Chem. Soc., 1990, 112, 7001-7031.
- K.C. Nicolaou, D. Vourloumis, N. Wissinger, and P. S. Baran, Angew. Chem. Int. Ed., 2000, 39, 44-122.

Comments