Bongkrekic Acid
Previously in Totally Synthesised, a chemical compound called tetrodotoxin from a puffer fish makes a plate of deadly sashimi from Japan. This time a delicacy from Indonesia known as tempe bongkrek and it is made from a fermented coconut press cake. However, a contaminated tempe bongkrek with bacterium Burkholderia cocovenans causes this food becomes toxic due to a chemical produced by the bacterium which is bongkrek acid or bongkrekic acid.
Tempe bongkrek is a well-known food from Java, especially in Banyumas Region, Central Java. As mentioned earlier, tempe bongkrek is made usually from a fermented coconut press cake by Rhizopus fungus. The contaminated tempe bongkrek contains bongkrekic acid and toxoflavion which are produced by B. cocovenans and this makes highly toxic tempe bongkrek. In the past, fatalities due to tempe bongkrek were common and it forced the government to ban its sale by law.
The mode of action of bongkrek acid is by binding to the adenine nucleotide translocator (ANT) from inside mitochondria and fixing its conformation, BKA inhibits the mitochondrial permeability transition (MPT) pore. This means, ATP cannot be exported out from mitochondria. Besides that, BKA is also found to inhibit mitochondrial-induced apoptosis by preventing the formation of MPT.
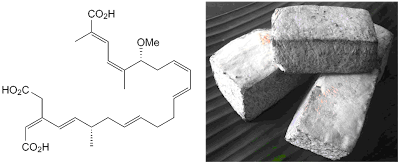
Bongkrek acid (BKA) has a unique structure with several double bonds that has 2 conjugated system and 2 stereocentres. This structure makes unique challenges to synthesise with selective configuration of the molecule. One of the interesting attempts to synthesise this unique molecule was done by Shindo's group from Kyushu University in Japan. This group synthesised by sticking 3 major fragments by Kocienski-Julia olefination and Suzuki-Miyaura coupling.
The key reaction in the synthesis of fragment A is the formation of (Z)-iodoalkane 3 from alkyne 2 TBS protection, homologation with paraformaldehyde and reduction by red-Al and I2.
Alkene 3 was then step-wise oxidised by MnO2 then NaClO2 to form the carboxylic acid. The MOM functional group is the most suitable protecting group due to in basic condition for deprotection it can isomerise to the unwanted (E)-BKA.
The synthesis of fragment B was started by asymmetric alkylation Evans oxazolidinone 8 with 7, which is prepared from 6 in 5 steps. This step produces 99% diastereomic excess (de) which means a very high yield and this is important for producing a selective configuration of BKA. Reductive removal of chemical auxillary followed by TBDPS protection gives 10 which then subjected to Mitsunobu reaction followed by oxidation to give tetrazolylsulfonyl 11, functionalised for Julia olefination. After deprotection of TBDPS, aldehyde 12 is formed under Swern condition which then finish off with CrCl2-boronalkenylation to functionalised middle fragment.
Fragment C consists of conjugated diene with MOM ester, (Z)-alkene, and stereocentre. The first step of the synthesis of fragment C was the epoxide 15 ring opening with lithium acetylide of MPM protected propargyl alcohol to give 16. Hydogenation of 16 using Lindlar catalyst gives selectively a (Z)-alkene which then followed by deprotection of TBS. Then, alkyne 18 was formed by oxidation of 17 under Swern condition followed by Corey-Fuchs alkynylation after carboxylation of the terminal alkyne 18. The selective conjugate addition of 18 with MeLi and CuI gives a (E)-alkene. Then, successive treatment with DIBAL and MnO2 gives trisubstituted alkene 19. The attempt to form 20 via Wittig reagent bearing MOM could not be used, possibly due to its instability. Therefore, to address this problem Shindo's group used torquoselective olefination via ynolate followed by formation of MOM ester. The synthesis of fragment C was finished off by deprotection of DDQ and oxidation to give fragment C 21.
With those three fragments are in place, then those three fragments can be put together to give BKA 1.
The first step was Kocienski-Julia olefination of fragment C 21 and fragment B 14 in the presence of KHMDS to give 22 (fragment B-C) with excellent E-selectivity and high yield. Then, 22 was coupled with fragment A 5 using Suzuki-Miyaura coupling to give the entire skeleton of BKA in high yield. The total synthesis of BKA was completed by straightforward primary alcohol using Jones reagent and acid deprotection of MOM ester to give BKA.
In this synthesis, by using three-component convergent strategy involving Kocienski-Julia olefination and Suzuki-Miyaura coupling, it shortens the steps into 18 steps (compare to earlier synthesis with 32 steps) and also has higher yield (about 6.4%).
References
E. J. Corey and A. Tramontano, J. Am. Chem. Soc., 1984, 106, 462.
Y. Sato, Y. Aso, and M. Shindo, Tetrahedron Letters, 2009, 50, 4164.
 |
| Bongkrek Acid and Tempe Bongkrek |
Tempe bongkrek is a well-known food from Java, especially in Banyumas Region, Central Java. As mentioned earlier, tempe bongkrek is made usually from a fermented coconut press cake by Rhizopus fungus. The contaminated tempe bongkrek contains bongkrekic acid and toxoflavion which are produced by B. cocovenans and this makes highly toxic tempe bongkrek. In the past, fatalities due to tempe bongkrek were common and it forced the government to ban its sale by law.
The mode of action of bongkrek acid is by binding to the adenine nucleotide translocator (ANT) from inside mitochondria and fixing its conformation, BKA inhibits the mitochondrial permeability transition (MPT) pore. This means, ATP cannot be exported out from mitochondria. Besides that, BKA is also found to inhibit mitochondrial-induced apoptosis by preventing the formation of MPT.
Bongkrek acid (BKA) has a unique structure with several double bonds that has 2 conjugated system and 2 stereocentres. This structure makes unique challenges to synthesise with selective configuration of the molecule. One of the interesting attempts to synthesise this unique molecule was done by Shindo's group from Kyushu University in Japan. This group synthesised by sticking 3 major fragments by Kocienski-Julia olefination and Suzuki-Miyaura coupling.
The key reaction in the synthesis of fragment A is the formation of (Z)-iodoalkane 3 from alkyne 2 TBS protection, homologation with paraformaldehyde and reduction by red-Al and I2.
 |
| Synthesis of fragment A |
 |
| Synthesis of fragment B |
 |
| Synthesis of fragment C |
With those three fragments are in place, then those three fragments can be put together to give BKA 1.
The first step was Kocienski-Julia olefination of fragment C 21 and fragment B 14 in the presence of KHMDS to give 22 (fragment B-C) with excellent E-selectivity and high yield. Then, 22 was coupled with fragment A 5 using Suzuki-Miyaura coupling to give the entire skeleton of BKA in high yield. The total synthesis of BKA was completed by straightforward primary alcohol using Jones reagent and acid deprotection of MOM ester to give BKA.
In this synthesis, by using three-component convergent strategy involving Kocienski-Julia olefination and Suzuki-Miyaura coupling, it shortens the steps into 18 steps (compare to earlier synthesis with 32 steps) and also has higher yield (about 6.4%).
References
E. J. Corey and A. Tramontano, J. Am. Chem. Soc., 1984, 106, 462.
Y. Sato, Y. Aso, and M. Shindo, Tetrahedron Letters, 2009, 50, 4164.


Comments