Physical Properties of Polymers
Polymers are well known for its wide range of application in daily life. One of the main factor that determine its application is by tuning its physical properties. This section will discuss about the properties in solution and in solid state, especially about melting temperature Tm and glass transition temperature Tg. Lastly, we will see the physical properties of elastomers.
As we know, polymer is a macromolecule with a long extended chain but in solution it does not form an extended chain. However, most polymers exist as random coils in dilute solution and this process is entropically driven due to more conformations are possible in this state. Besides that, in thermodynamically stable ideal solution, the radius of this random coil or gyration radius Rg can be quantified as followed.
Where n is the number of monomer per unit of chain (Dp) and L is the length of single monomer unit. For example, for polyethylene with n = 10 000 and L is 0.154 nm, the Rg is around 22 nm; hence polymer coils touch at solution with concentration around 0.7 %. Besides that, Rg is much shorter than the whole extended chain (c.f. 1260 nm) which implies the random coil conformation is much more compact that the extended chain.
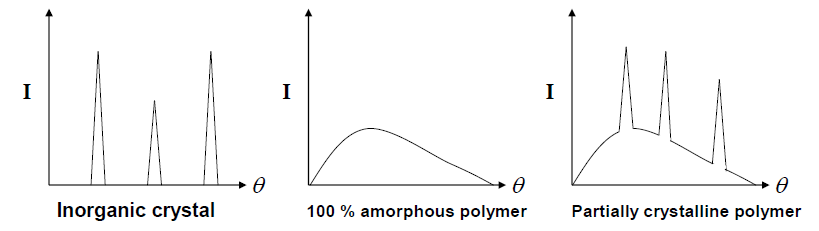
In solid state, polymers can have 2 main domains in macroscopic level, crystalline and amorphous region. Amorphous region consists of random coils while the crystalline region is a well-packed polymer chains region. This well-packed region makes the density of amorphous polymer is lower than crystalline polymer. Besides that, it is possible to create a 100 % of amorphous polymer but it is impossible to create 100 % crystalline polymer. The process of making 100 % crystalline polymer is kinetically impossible. One way to quantify the level of crystallinity of a polymer in solid state is via degree of crystallinity Dc and this value can be determined from the data of X-ray diffraction or IR spectroscopy is also applicable for same cases. Considering Bragg's Law
the diffraction intensity of an amorphous and partially crystalline polymer is shown below with the comparison of an inorganic crystal.
Hence, Dc can be calculated as followed.
In general, there are 3 ways to achieve high crystalline polymer and they are
Now, considering a case of polyethylene (PE) and polypropylene (PP) which both of them can achieve Dc as high as 70 %. When both monomers form a poly(ethylene-stat-propylene), Dc decreases dramatically to near amorphous state (~0%).
This amorphous state of copolymer is due to random distribution of methyl group in PP which leads to an irregular structure, hence Dc is reduced.
Another case is shown below for these two polymers.
Both polymers can achieve high crystallinity and this is due to the C=O group has the same size of CH2 so it only causes a small disruption in packing system.
One of the main characters of crystalline polymers is a temperature where it lose its crystallinity or the crystalline region become disordered. This temperature is called melting temperature Tm and this temperature is relatively broad (up to 30 - 40 °C). Besides that, Tm depends on the same factor as Dc where aromatic spacer group and strong interaction forces would cause high Tm.
However, Tm is only applicable for at least partially crystalline; hence not all polymers have Tm.
Another value that can be used to observe the physical properties of polymers is glass transition temperature Tg and this time is applicable to all polymers. Tg can be defined as the temperature onset of significant segmental chain motion which means Tg does not involve translational motion. When a polymer in a temperature above Tg, it will have a rubbery characteristic; while the temperature is below Tg it will be brittle.
The structure of polymers could affect Tg as demonstrated below.
From the data above, Tg decreases as the length of R' increases but increasing R' again the changes in Tg would be less prominent as the polymers become waxy. Meanwhile, poly(methacrylates) has higher Tg than poly(acrylates) due to Me group at the backbone causes greater stiffness to the chain.
During the transition temperature, it will always be accompanied by various physical changes such as specific volume (reciprocal value of density), refractive index, specific heat etc. Considering the example below of a plot between specific volume of amorphous polymer (1) and partially crystalline polymer (2).
One important point to note from that Tg is always lower than Tg (around 0.5 - 0.7Tm). Another method to determine Tg is via differential scanning calorimetry (DSC). In this technique, the sample is heated above its Tg and then it is allowed to cool down at different rates.
Tuning Tg is important to determine the application of polymers and this process can be achieved by making a stat-polymer which allows raise or lower the temperature or adding plasticiser which would lower Tg. The new Tg of copolymer (Tg*) can be calculated using Fox equation.
Where Tgn is Tg's of the homopolymer (in Kelvin) and Fn is the weight fraction of component n. In this equation, it is assumed that there is no interactions between comonomers or the polymer and plasticiser.
The last important properties of polymers is its mechanical properties. The rubber elasticity is a unique property to polymer and an elastic polymer is called elastomer.The properties of elastomers are
To achieve these properties, an elastomer must have
The network of cross-link would help the elastomer to retract rapidly and recover to its original dimension. One of the application of cross-linkers is vulcanisation process in the tyre manufacture.
Typically, in a cross-linker network there are 20 to 50 sulphur atoms per cross-link.
As we know, polymer is a macromolecule with a long extended chain but in solution it does not form an extended chain. However, most polymers exist as random coils in dilute solution and this process is entropically driven due to more conformations are possible in this state. Besides that, in thermodynamically stable ideal solution, the radius of this random coil or gyration radius Rg can be quantified as followed.
Where n is the number of monomer per unit of chain (Dp) and L is the length of single monomer unit. For example, for polyethylene with n = 10 000 and L is 0.154 nm, the Rg is around 22 nm; hence polymer coils touch at solution with concentration around 0.7 %. Besides that, Rg is much shorter than the whole extended chain (c.f. 1260 nm) which implies the random coil conformation is much more compact that the extended chain.
In solid state, polymers can have 2 main domains in macroscopic level, crystalline and amorphous region. Amorphous region consists of random coils while the crystalline region is a well-packed polymer chains region. This well-packed region makes the density of amorphous polymer is lower than crystalline polymer. Besides that, it is possible to create a 100 % of amorphous polymer but it is impossible to create 100 % crystalline polymer. The process of making 100 % crystalline polymer is kinetically impossible. One way to quantify the level of crystallinity of a polymer in solid state is via degree of crystallinity Dc and this value can be determined from the data of X-ray diffraction or IR spectroscopy is also applicable for same cases. Considering Bragg's Law
the diffraction intensity of an amorphous and partially crystalline polymer is shown below with the comparison of an inorganic crystal.
Hence, Dc can be calculated as followed.
In general, there are 3 ways to achieve high crystalline polymer and they are
- regular chain structure (e.g. isotactic or syndiotactic polymers),
- stiff chain (e.g. aromatic spacer group or conjugated system),
- strong interaction forces between each chains.
Now, considering a case of polyethylene (PE) and polypropylene (PP) which both of them can achieve Dc as high as 70 %. When both monomers form a poly(ethylene-stat-propylene), Dc decreases dramatically to near amorphous state (~0%).
This amorphous state of copolymer is due to random distribution of methyl group in PP which leads to an irregular structure, hence Dc is reduced.
Another case is shown below for these two polymers.
Both polymers can achieve high crystallinity and this is due to the C=O group has the same size of CH2 so it only causes a small disruption in packing system.
One of the main characters of crystalline polymers is a temperature where it lose its crystallinity or the crystalline region become disordered. This temperature is called melting temperature Tm and this temperature is relatively broad (up to 30 - 40 °C). Besides that, Tm depends on the same factor as Dc where aromatic spacer group and strong interaction forces would cause high Tm.
However, Tm is only applicable for at least partially crystalline; hence not all polymers have Tm.
Another value that can be used to observe the physical properties of polymers is glass transition temperature Tg and this time is applicable to all polymers. Tg can be defined as the temperature onset of significant segmental chain motion which means Tg does not involve translational motion. When a polymer in a temperature above Tg, it will have a rubbery characteristic; while the temperature is below Tg it will be brittle.
The structure of polymers could affect Tg as demonstrated below.
From the data above, Tg decreases as the length of R' increases but increasing R' again the changes in Tg would be less prominent as the polymers become waxy. Meanwhile, poly(methacrylates) has higher Tg than poly(acrylates) due to Me group at the backbone causes greater stiffness to the chain.
During the transition temperature, it will always be accompanied by various physical changes such as specific volume (reciprocal value of density), refractive index, specific heat etc. Considering the example below of a plot between specific volume of amorphous polymer (1) and partially crystalline polymer (2).
One important point to note from that Tg is always lower than Tg (around 0.5 - 0.7Tm). Another method to determine Tg is via differential scanning calorimetry (DSC). In this technique, the sample is heated above its Tg and then it is allowed to cool down at different rates.
Tuning Tg is important to determine the application of polymers and this process can be achieved by making a stat-polymer which allows raise or lower the temperature or adding plasticiser which would lower Tg. The new Tg of copolymer (Tg*) can be calculated using Fox equation.
Where Tgn is Tg's of the homopolymer (in Kelvin) and Fn is the weight fraction of component n. In this equation, it is assumed that there is no interactions between comonomers or the polymer and plasticiser.
The last important properties of polymers is its mechanical properties. The rubber elasticity is a unique property to polymer and an elastic polymer is called elastomer.The properties of elastomers are
- must stretch rapidly to high elongation with little energy dissipated,
- must have high tensile strength and stiffness when fully extended,
- must retract rapidly,
- must recover to its original dimension.
To achieve these properties, an elastomer must have
- high molecular weight for mechanical strength,
- for high segmental mobility, the temperature must be above Tg,
- must be amorphous in unstretched state,
- must have a network of cross-links.
The network of cross-link would help the elastomer to retract rapidly and recover to its original dimension. One of the application of cross-linkers is vulcanisation process in the tyre manufacture.












Comments