Synthesis of Heteroaromatic Compounds: 5-Membered Rings
Heteroaromatic compounds are commonly found in pharmaceutical, agrochemicals and many known chemicals in the world. In this section, we will see how heteroaromatic compounds can be synthesised and if we examine closely the key reactions in making heteroaromatic compounds are the carbonyls chemistry.
As mentioned earlier, the key reactions of heteroaromatics synthesis are the carbonyls chemistry. If we see the structure of N-heteroaromatic or O-heteroaromatic, it resembles the enamine type structure and enol ether type structure.
From the diagram above, we can see that all the structures start from a carbonyls chemistry. Besides that, the reactivity of enamine or imine can be used to build a diketones which are essential for heteroaromatic synthesis.
Now, we can start to build a heteroaromatic and we will start from the simplest units which are the 5-membered rings.
Pyrrole has an enamine type structure and if we do the analysis as shown earlier, pyrrole can be synthesis from 1,4 diketone and a primary amine.
This synthesis of pyrrole is known as Paal-Knorr synthesis, named after two German chemists Carl Paal and Ludwig Knorr.
The reaction proceeds via:
Paal-Knorr reaction can also be used for furan and tiophene synthesis. The synthesis of furan can be done in similar way by reacting 1,4 diketone with acid and tiophene can be synthesised from 1,4 diketone and H2S or P2S5 or Lawesson's reagent (P4S10).
If we see the Paal-Knorr reactions, they have a common feature which is 1,4-diketone as starting material and 1,4- diketone can be synthesised using enamine chemistry as shown below.
Another alternative synthesis of pyrrole which is known as Knorr synthesis is shown below.
The reaction proceeds via:
5-membered heteroaromatics could also have more than 1 heteroatom such as imidazole, thiazole and oxazole.
Those heteroaromatics have 1,3-diheteroatoms. Thiazole can be synthesised by analysing hard-base nucleophilicity; hence the synthesis of thiazole is shown below.
The reaction proceeds via:
Furthermore, imidazole can also be synthesised using the same method but oxazole can be made using this method due O atom on amide is not nucleophilic enough.
Oxazole can be synthesised using the method as shown below.
Pyridine or triethylamine (Et3N) is commonly used for this synthesis.
For 1,2-diheteroatom such as isoxazole and pyrazole can be synthesised from 1,3-diketone and hydroxylame for isoxazole or hydrazine for pyrazole.
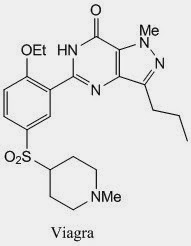
1,3-diketone can also be synthesised from an enamine; and this time acyl chloride is used. This imidazole reaction is also used in the synthesis of slidenafil which is the active component of viagra.
In isoxazole synthesis, a problem of regioselectiviy can occur when unsymmetrical dicarbonyl is used. The selectivity on this reaction is the N atom is more nucleophilic than O atom; hence N atom would react with the more electrophile carbonyl.
Isoxazoles could also be synthesised using cycloaddition between nitrile oxides and an alkynes via [3+2] reaction.
Furthermore, there are two ways to synthesise nitrile oxides as shown below.
Cycloaddition reaction can also be used in the synthesis of triazoles where an alkyne is reacted with an azide.
The triazole synthesis is known as "click reaction" where the reaction is catalysed by Cu(I) halide and this reaction can be done in water solvent.
This reaction also work without R group attach to azide and this can be used to synthesise tetrazole.
Tetrazole can be used as substitute of carboxylic acid in drugs such as in indomethacin.
As mentioned earlier, the key reactions of heteroaromatics synthesis are the carbonyls chemistry. If we see the structure of N-heteroaromatic or O-heteroaromatic, it resembles the enamine type structure and enol ether type structure.
From the diagram above, we can see that all the structures start from a carbonyls chemistry. Besides that, the reactivity of enamine or imine can be used to build a diketones which are essential for heteroaromatic synthesis.
Now, we can start to build a heteroaromatic and we will start from the simplest units which are the 5-membered rings.
Pyrrole has an enamine type structure and if we do the analysis as shown earlier, pyrrole can be synthesis from 1,4 diketone and a primary amine.
This synthesis of pyrrole is known as Paal-Knorr synthesis, named after two German chemists Carl Paal and Ludwig Knorr.
The reaction proceeds via:
Paal-Knorr reaction can also be used for furan and tiophene synthesis. The synthesis of furan can be done in similar way by reacting 1,4 diketone with acid and tiophene can be synthesised from 1,4 diketone and H2S or P2S5 or Lawesson's reagent (P4S10).
If we see the Paal-Knorr reactions, they have a common feature which is 1,4-diketone as starting material and 1,4- diketone can be synthesised using enamine chemistry as shown below.
Another alternative synthesis of pyrrole which is known as Knorr synthesis is shown below.
The reaction proceeds via:
5-membered heteroaromatics could also have more than 1 heteroatom such as imidazole, thiazole and oxazole.
Those heteroaromatics have 1,3-diheteroatoms. Thiazole can be synthesised by analysing hard-base nucleophilicity; hence the synthesis of thiazole is shown below.
Furthermore, imidazole can also be synthesised using the same method but oxazole can be made using this method due O atom on amide is not nucleophilic enough.
Oxazole can be synthesised using the method as shown below.
Pyridine or triethylamine (Et3N) is commonly used for this synthesis.
For 1,2-diheteroatom such as isoxazole and pyrazole can be synthesised from 1,3-diketone and hydroxylame for isoxazole or hydrazine for pyrazole.
1,3-diketone can also be synthesised from an enamine; and this time acyl chloride is used. This imidazole reaction is also used in the synthesis of slidenafil which is the active component of viagra.
In isoxazole synthesis, a problem of regioselectiviy can occur when unsymmetrical dicarbonyl is used. The selectivity on this reaction is the N atom is more nucleophilic than O atom; hence N atom would react with the more electrophile carbonyl.
Isoxazoles could also be synthesised using cycloaddition between nitrile oxides and an alkynes via [3+2] reaction.
Furthermore, there are two ways to synthesise nitrile oxides as shown below.
Cycloaddition reaction can also be used in the synthesis of triazoles where an alkyne is reacted with an azide.
The triazole synthesis is known as "click reaction" where the reaction is catalysed by Cu(I) halide and this reaction can be done in water solvent.
This reaction also work without R group attach to azide and this can be used to synthesise tetrazole.
Tetrazole can be used as substitute of carboxylic acid in drugs such as in indomethacin.























Comments