Organic Chemistry for A-Level: Polymers
In this final section for organic chemistry for A-level, we will see the chemistry of polymers. This section will discuss the basic principles in polymer chemistry, including type of polymers and polymerisation reaction. This section will be concluded by extension section about inorganic polymers.
Polymers can be found wandering around in our daily life from plastic bottles to carrier bags. Those "plasticy" things are mainly synthetic polymers. In the other sides, nature also produces some polymers as well such as natural rubber, protein and DNA; hence they are called natural polymers. The word polymer itself is derived from ancient greek words, poly from polus meaning many and mer from meros meaning parts. Therefore, polymers can be defined as molecule that consists of many repeating parts or unit. For example, Polyethylene or PE is a polymer because it consists of repeating unit of ethylene (ethene) which is the monomer. Besides that, PE is made from single monomer which is ethylene so it is called a homopolymer. However, polymers can also be made from different monomers such as in ABS which is made from monomers of acrylonitrile, 1,3-butadiene and styrene; hence it is called co-polymer.
The process of making polymers is called polymerisation and general there are two main types of polymerisation, free radical polymerisation and condensation polymerisation. In free radical polymerisation, it involves radical species to polymerise the polymer and in general there are 4 steps in radical polymerisation.
The first step is dissociation step where the initiator is dissociated to form radical species which would initiate the reaction. The dissociation of initiator molecule can be done by thermal reaction (using heat) or irradiate the molecule using UV light. Common initiator molecules are peroxide-based or aza-based molecule due to the weak O-O and N=N bond which can be broken thermally or by UV light. Aza-based molecule can produce a stable nitrogen gas which is the driving force of the radical formation.
Then, the formed radical attack the monomer to start the polymerisation. This step is called initiation process and this reaction would start the polymerisation. Then, the radical monomer can attack another monomers to elongate the polymer chain. Because in this process the polymeric chain is getting longer, so this step is called propagation step. The polymerisation would stop when polymeric radical chains form a stable polymer chain, so this process is called termination process because in this step the "living" polymeric chain is used to form the final product. Free radical chain is commonly used to form a polymer from a vinyl monomer or monomers with C=C bond. The steps of free radical polymerisation can be summarised as followed.
If we see closely the free radical polymerisation, it starts with a monomer with C=C bond and the product is a polyalkene. Therefore, this reaction is commonly known as addition polymerisation. In the example above of polystyrene (PS), the tetrahedral backbone can cause different arrangement of phenyl groups or known as tacticity. The first possible arrangement is all the phenyl groupd (or side chain) is arranged in the same way or regular structure. This structure is called isotactic and it will produce a rigid, tough and heat resistant polymer due to efficient packing of the side chain.
This type of polymers is commonly used as food container or hospital equipments.
The second possible arrangement is the side chain alternates between one side and others and this is called syndiotactic. This arrangement is still a regular structure.
The last possible arrangement is the side chain arrange themselves randomly which makes the structure more flexible and softer polymers due to less efficient packing of the side chain. This arrangement is called atactic.
The second type of polymerisation is called condensation polymerisation due to the production of a small molecule, commonly water, or known as condensate. Condensation polymerisation is commonly used for the formation of polyesters and polyamides. In these cases, it requires a difunctional monomers to allow the formation of polymers.
Polyesters can be made from dicarboxylic acids and diols, and the side product of this polymerisation is water. One of the common polyesters in daily life is Poly(ethylene terephatalate) or known as PET which is used as plastic bottles. The synthesis of PET is done by reacting phtalic acid and ethane-1,2-diol under acidic conditions.
The rigid structure of PET is due to the flat benzene ring which can contribute to very efficient packing of the polymeric chain. However, this property cause a bit of environmental problem due to difficulty to degrade PET, recycling PET bottle is necessary. One of the example is one of the great manufacturers of football shirt made the football shirt for England team in World Cup 2014 from a recycled plastic bottle.
Then, polyamides as one of the commonest polymer and this type of condensation polymers has an amide linkage to connect each monomers. One of the great example of natural polyamides is protein which has many several functions in our body. One of the example of below is botulinum toxin which is produced by Clostridium botulinum and it is known as the most toxic substance in the world.
Synthetic polyamides is commonly made from diacid chloride or dicarboxylic acid and diamine. One of the example of synthetic polyamides is Nylon. In general, there are two types of Nylon; Nylon-6 and Nylon-6,6. Nylon-6 is synthesised from a difunctional monomer of 6-aminohexanoic acid which forms an intermediate of a cyclic caprolactam which then undergoes polymerisation.
Meanwhile, Nylon-6,6 is synthesised from hexane-1,6-dioic acid and hexane-1,6-diamine.
This long alkyl chain in both Nylon-6 and Nylon-6,6 gives the flexibility of Nylon but it is still a strong polymer due to hydrogen bonding interaction. This interaction is absent in polyesters.
Plastic manufacturers owe a lot from these processes but sometimes they need to tweak the polymers so it fit with the markets or the requirement such as its hardness or hydrophobicity. These properties can be tuned by changing the structure of polymers. In this case, we will try to see how to make the polymers harder and generally there are three approaches that can be done such as:
Hardening the polymers by using cross-linkers can be seen from tyres against natural rubber. Both polymers are polyisoprene.
In tyres manufacture, the cross-linker is achieved by adding sulphur into a mixture which makes each polymeric chain is connected by disulphide bridges. This process is known as vulcanisation process and it was developed by Charles Goodyear in 19th century. The name of vulcanisation was derived from the Roman god of fire, Vulcan.
The strong disulphide bridges keep the shape of polyisoprene when external force is applied to the polymer. This type of polymer with cross-linkers are commonly known as elastomers.
Another way to generate to cross-linked polymers is by manipulating the monomers to have multiple points for polymerisation. This strategy is used in the making of contact lenses as the monomer below can be polymerised at both end to form a polymer network which is a hydrogels. In this mixture, a monofunctional monomers is also introduced as a capping agent.
The second strategy is to reduce the chain length of monomers and this can be seen in polyurethanes for pacemaker.
The polymeric chain of this polyurethane consist of two units. The red unit has longer alkyl unit than the blue unit which makes the red unit will provides the rigidity of the pacemaker while the blue unit provides the flexibility of the pacemaker. The flexibility of the red unit is due to the free rotation around C-C carbon in both red and blue units. This combination would give the pacemaker the desired property which is a flexible but durable polymer.
The third strategy to harden the polymers is by using the aromatic monomers such as in Kevlar against Nylon.
Kevlar is commonly used as bulletproof jacket which requires a hard property while Nylon is commonly used for stockings or socks which requires flexibility. The hardness of Kevlar comes from the fact that both diacid and diamine in Kevlar are aromatic compounds. These planar structure of benzene ring would give a better packing of the polymeric chain and also the fact that there is no bond rotation around C-C aromatic bond. Meanwhile, Nylon would have the free rotation around C-C bond which gives it the flexibility.
Extension Topic: Silicone
Non carbon-based polymers are available in our daily life and one of the example is silicone. Silicone is a Si-based polymer which is derived from silicon dichloride compounds. One of the example of silicone is poly(dimethylsiloxane) which is known as PDMS. This polymer can be synthesised from hydrolysis reaction as shown below.
This polymer can be capped other Si-based compound such as trimethylsilyl chloride.
PDMS is a hydrophobic liquid polymer with high flexibility chains due to the alternating structure of Si and O. Hence, PDMS is commonly used from oil lubricant to shampoo. PDMS is known as dimethicone is shampoo formulation (more information about chemistry of shampoo, see here).
Another use of Si-based polymers is in contact lenses as a silicone hybrid polymers. In this polymer, PDMS is combined with C-based monomers to give the properties of contact lenses as shown below.
In this case, PDMS serves to give oxygen clarity, poly(ethylene oxide) or the red unit has a function to maintain clarity while vinyl unit (blue and green unit) gives the hydrophilicity properties for comfort and resistance to lipids and proteins.
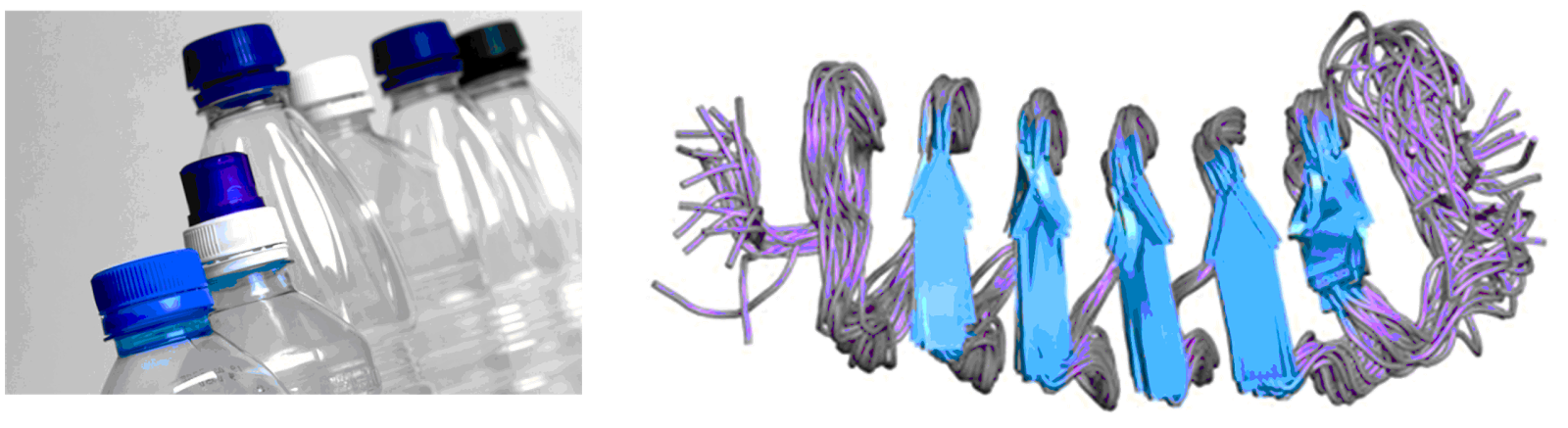 |
| Synthetic vs Natural Polymers Plastic Bottle vs Antifreeze protein from Tenebrio molitor |
 |
| Example of homopolymer and copolymer |
The first step is dissociation step where the initiator is dissociated to form radical species which would initiate the reaction. The dissociation of initiator molecule can be done by thermal reaction (using heat) or irradiate the molecule using UV light. Common initiator molecules are peroxide-based or aza-based molecule due to the weak O-O and N=N bond which can be broken thermally or by UV light. Aza-based molecule can produce a stable nitrogen gas which is the driving force of the radical formation.
Then, the formed radical attack the monomer to start the polymerisation. This step is called initiation process and this reaction would start the polymerisation. Then, the radical monomer can attack another monomers to elongate the polymer chain. Because in this process the polymeric chain is getting longer, so this step is called propagation step. The polymerisation would stop when polymeric radical chains form a stable polymer chain, so this process is called termination process because in this step the "living" polymeric chain is used to form the final product. Free radical chain is commonly used to form a polymer from a vinyl monomer or monomers with C=C bond. The steps of free radical polymerisation can be summarised as followed.
If we see closely the free radical polymerisation, it starts with a monomer with C=C bond and the product is a polyalkene. Therefore, this reaction is commonly known as addition polymerisation. In the example above of polystyrene (PS), the tetrahedral backbone can cause different arrangement of phenyl groups or known as tacticity. The first possible arrangement is all the phenyl groupd (or side chain) is arranged in the same way or regular structure. This structure is called isotactic and it will produce a rigid, tough and heat resistant polymer due to efficient packing of the side chain.
This type of polymers is commonly used as food container or hospital equipments.
The second possible arrangement is the side chain alternates between one side and others and this is called syndiotactic. This arrangement is still a regular structure.
The last possible arrangement is the side chain arrange themselves randomly which makes the structure more flexible and softer polymers due to less efficient packing of the side chain. This arrangement is called atactic.
The second type of polymerisation is called condensation polymerisation due to the production of a small molecule, commonly water, or known as condensate. Condensation polymerisation is commonly used for the formation of polyesters and polyamides. In these cases, it requires a difunctional monomers to allow the formation of polymers.
Polyesters can be made from dicarboxylic acids and diols, and the side product of this polymerisation is water. One of the common polyesters in daily life is Poly(ethylene terephatalate) or known as PET which is used as plastic bottles. The synthesis of PET is done by reacting phtalic acid and ethane-1,2-diol under acidic conditions.
The rigid structure of PET is due to the flat benzene ring which can contribute to very efficient packing of the polymeric chain. However, this property cause a bit of environmental problem due to difficulty to degrade PET, recycling PET bottle is necessary. One of the example is one of the great manufacturers of football shirt made the football shirt for England team in World Cup 2014 from a recycled plastic bottle.
Then, polyamides as one of the commonest polymer and this type of condensation polymers has an amide linkage to connect each monomers. One of the great example of natural polyamides is protein which has many several functions in our body. One of the example of below is botulinum toxin which is produced by Clostridium botulinum and it is known as the most toxic substance in the world.
 |
| Crystal structure of botulinum toxin |
Meanwhile, Nylon-6,6 is synthesised from hexane-1,6-dioic acid and hexane-1,6-diamine.
This long alkyl chain in both Nylon-6 and Nylon-6,6 gives the flexibility of Nylon but it is still a strong polymer due to hydrogen bonding interaction. This interaction is absent in polyesters.
Plastic manufacturers owe a lot from these processes but sometimes they need to tweak the polymers so it fit with the markets or the requirement such as its hardness or hydrophobicity. These properties can be tuned by changing the structure of polymers. In this case, we will try to see how to make the polymers harder and generally there are three approaches that can be done such as:
- using cross-linker,
- shortening the monomeric chain,
- using aromatic functional group.
Hardening the polymers by using cross-linkers can be seen from tyres against natural rubber. Both polymers are polyisoprene.
In tyres manufacture, the cross-linker is achieved by adding sulphur into a mixture which makes each polymeric chain is connected by disulphide bridges. This process is known as vulcanisation process and it was developed by Charles Goodyear in 19th century. The name of vulcanisation was derived from the Roman god of fire, Vulcan.
The strong disulphide bridges keep the shape of polyisoprene when external force is applied to the polymer. This type of polymer with cross-linkers are commonly known as elastomers.
Another way to generate to cross-linked polymers is by manipulating the monomers to have multiple points for polymerisation. This strategy is used in the making of contact lenses as the monomer below can be polymerised at both end to form a polymer network which is a hydrogels. In this mixture, a monofunctional monomers is also introduced as a capping agent.
 |
| Hydrogel monomer |
The second strategy is to reduce the chain length of monomers and this can be seen in polyurethanes for pacemaker.
The polymeric chain of this polyurethane consist of two units. The red unit has longer alkyl unit than the blue unit which makes the red unit will provides the rigidity of the pacemaker while the blue unit provides the flexibility of the pacemaker. The flexibility of the red unit is due to the free rotation around C-C carbon in both red and blue units. This combination would give the pacemaker the desired property which is a flexible but durable polymer.
The third strategy to harden the polymers is by using the aromatic monomers such as in Kevlar against Nylon.
Kevlar is commonly used as bulletproof jacket which requires a hard property while Nylon is commonly used for stockings or socks which requires flexibility. The hardness of Kevlar comes from the fact that both diacid and diamine in Kevlar are aromatic compounds. These planar structure of benzene ring would give a better packing of the polymeric chain and also the fact that there is no bond rotation around C-C aromatic bond. Meanwhile, Nylon would have the free rotation around C-C bond which gives it the flexibility.
Extension Topic: Silicone
Non carbon-based polymers are available in our daily life and one of the example is silicone. Silicone is a Si-based polymer which is derived from silicon dichloride compounds. One of the example of silicone is poly(dimethylsiloxane) which is known as PDMS. This polymer can be synthesised from hydrolysis reaction as shown below.
This polymer can be capped other Si-based compound such as trimethylsilyl chloride.
PDMS is a hydrophobic liquid polymer with high flexibility chains due to the alternating structure of Si and O. Hence, PDMS is commonly used from oil lubricant to shampoo. PDMS is known as dimethicone is shampoo formulation (more information about chemistry of shampoo, see here).
Another use of Si-based polymers is in contact lenses as a silicone hybrid polymers. In this polymer, PDMS is combined with C-based monomers to give the properties of contact lenses as shown below.
In this case, PDMS serves to give oxygen clarity, poly(ethylene oxide) or the red unit has a function to maintain clarity while vinyl unit (blue and green unit) gives the hydrophilicity properties for comfort and resistance to lipids and proteins.
















Comments