The Chemistry of Carbonyls: The Reaction at the α-Carbon
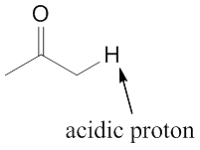
In this section, we will unravel one of the prominent properties of carbonyl compound which involves the α-carbon. The α-carbon of carbonyl compounds has unique properties which is the α-H is slightly acidic. This allowed a distinct reaction of carbonyl compounds that does not have by another functional groups.
As mentioned in the introduction, the α-H in carbonyl compound is slightly acidic because its conjugate acid can delocalise the the negative charge into oxygen atom. This delocalised structure stabilise the anion to give its acidic property.
When the oxygen carries the negative charge, it forms the most stable form of conjugate and it is known as enolate. The enolate is key important intermediate reaction that we will encounter later on. The α-C would react with electrophile and this is the key step of the mechanism of the reaction in this section.
The first reaction that based on this enolate is the alkylation and the key of this reaction is to choose the proper base to deprotonate it. Let take the example if we choose the hydroxide ions as the base.
In this case pKc is 5 and this means the enolate form is less than the carbonyls. However, there is a better base that can be used to protonate the carbonyl which is lithium diisopropylamide (LDA).
The reaction of enolate with LDA gives pKc is -15 which means almost all the carbonyl turns into enolate, hence the reaction can happen. Then, to alkylate the carbonyl we need source of carbocation so alkyl halide can be used as it is a good source for carbocation as shown below.
Hence, to summarise the alkylation reaction as:
The second reaction is the reaction with halogens where the halogens act as electrophile. One of the famous reaction in this type is the reaction with I2 in basic condition. This reaction uses hydroxide ion as the base, so the consequence is only small concentration of enolates. This reaction produces yellow precipitate of iodoform (CHI3).
The iodoform is formed from the nucleophilic attack to the carbonyl carbon to form carboxylate ions, so this reaction can be said as oxidation reaction (oxidation level 2 to 3). This reaction is commonly used to identify methyl group in ketones.
The third reaction is aldol reaction where 2 carbonyl compounds are condensed. The product reaction of this reaction is C-C bond. The example of this reaction is the condensation of acetone.
This reaction is known as the aldol reaction, but the reaction does not stop until this step. The elimination of OH functional group to form a more stable product and this reaction is known as aldol condensation. Furthermore, this pathway is the minor pathway and E1cb because it occurs on the conjugate base (cb).
Another example is aldol reaction of 1-phenylethanone which form more stable compound because it has bigger conjugation system than the product of acetone's aldol reaction and condensation. This measure of the reaction is controlled under thermodynamic control because the main driving force of this reaction is the relative stability of the product with its reactant as shown below.
Another factor that controls the aldol reaction and condensation is the kinetics control which related to how fast the reaction is. This rate of reaction is determined by the reactivity of carbonyl compound as electrophiles. In this case, aldehydes are generally more reactive than ketones due to electronic donation and steric factor (see hydrate formation). The reactivity of the carbonyl compounds can be seen from the reaction mixed-aldol reaction and condensation as shown below.
Furthermore, all of the aldol condensations form an α-β unsaturated ketones which are a good electrophile as shown in its resonance structure.
Lastly, there is one variation of aldol reaction that involving α-β unsaturated ketones which is known as the Robinson annelation. This reaction is quite important as it forms one of the constituent of steroid skeleton.
The example of the Robinson annelation reaction to form ring A and B is shown below.
Then, the mechanism is shown below.
As mentioned in the introduction, the α-H in carbonyl compound is slightly acidic because its conjugate acid can delocalise the the negative charge into oxygen atom. This delocalised structure stabilise the anion to give its acidic property.
 |
| The delocalised structure of the conjugate base of acetone |
The first reaction that based on this enolate is the alkylation and the key of this reaction is to choose the proper base to deprotonate it. Let take the example if we choose the hydroxide ions as the base.
In this case pKc is 5 and this means the enolate form is less than the carbonyls. However, there is a better base that can be used to protonate the carbonyl which is lithium diisopropylamide (LDA).
The reaction of enolate with LDA gives pKc is -15 which means almost all the carbonyl turns into enolate, hence the reaction can happen. Then, to alkylate the carbonyl we need source of carbocation so alkyl halide can be used as it is a good source for carbocation as shown below.
 |
| Alkylation mechanism |
The second reaction is the reaction with halogens where the halogens act as electrophile. One of the famous reaction in this type is the reaction with I2 in basic condition. This reaction uses hydroxide ion as the base, so the consequence is only small concentration of enolates. This reaction produces yellow precipitate of iodoform (CHI3).
 |
| Iodoform formation (1) - the formation of 1,1,1-triiodopropanone |
 |
| Iodoform formation (2) |
The third reaction is aldol reaction where 2 carbonyl compounds are condensed. The product reaction of this reaction is C-C bond. The example of this reaction is the condensation of acetone.
 |
| Aldol reaction of acetone |
 |
| Aldol condensation of acetone |
 |
| Aldol reaction and condensation of 1-phenylethanone |
 |
| The mixed aldol condensation of benzaldehyde and acetone |
| The resonance structure of but-3-en-2-one |
Lastly, there is one variation of aldol reaction that involving α-β unsaturated ketones which is known as the Robinson annelation. This reaction is quite important as it forms one of the constituent of steroid skeleton.
 |
| Steroid skeleton |
 |
| The Robinson annelation |
 |
| The Robinson annelation mechanism |



Comments