In this section we will have a discussion about another reaction in carbonyl chemistry which is nucleophilic addition/elimination. The examples of this reaction is when carbonyl reacts with alcohol, or amine (either primary amine or secondary amine). Furthermore, the reaction of carbonyl with water form a hydrate which we will discuss more thoroughly later on. To start with, we will see the hydrate formation.
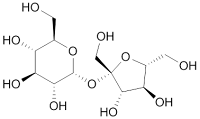 |
| Sucrose, an example of acetal |
Hydrate is formed when carbonyl react with water with helps either acid or base catalyst. The hydrate formation happens in equilibrium reaction and the acid or base catalyst does not change the equilibrium position. The catalyst only affects the rate of the hydrate formation. The reaction of carbonyl (propanone) with water in acid condition is shown below.
 |
| Hydrate formation under acid condition |
Meanwhile, in basic condition the mechanism is shown below.
 |
| Hydrate formation under basic condition |
Because the reaction happens in equilibrium, one of indicator that could tell us about how much carbonyls are converted into hydrate form is its equilibrium constant (K) which is shown below.
From K we could predict the relative stability of carbonyl compound with its hydrate form. If K is huge, so it means its hydrate form is higher concentration than the carbonyl compound in equilibrium condition. Therefore, we can say that the hydrate form is more stable product than the carbonyl compound. Hence, there are 2 factors that we can use to rationalise this case, which are steric factor and electronic effect. Let put ethanal as our starting point because it has K = 1.4, so it is around 58.3% hydrate in equilibrium.
If we have 2,2-dimethyl-propanal, the K = 0.25 which means only 20% of hydrate is formed at equilibrium position, which means the product is less stable than its reactant. Hence, to rationalise this case we have to see the structure of reactant and product first. When we have carbonyl compound we have sp2, but our product is a tetrahedral carbon (sp3). This means, we force a large group into more confined environment, therefore bigger group commonly stabilise the carbonyl compound. Therefore, propanone would have smaller K than ethanal; and propanone still have smaller K than 2,2-dimethyl-propanal. The reason in this case is based on the steric effect.
 |
| Steric effect on hydrate formation's equilibrium constant |
In the electronic effect, for example we have 1-chloropropanone, it has K = 0.1 which is higher than propanone. The reason behind this case is Cl is highly electronegative which means it increases the positive charge in C atom of C=O bond. Meanwhile, methyl group is electron-donating group, so it decreases the positive charge. A compound will be more stable if it has smaller charge; so by putting stronger electron-withdrawing group it destabilise the carbonyl compound, hence hydrate is formed in higher concentration.
Hence, those 2 factors that are generally considered to determine the relative stability of carbonyl compound to its hydrate form, or vice versa.
 |
| Electronic effect on hydrate formation's equilibrium |
Then, we can move to another reaction in carbonyl chemistry which is nucleophilic addition/elimination reaction. In this reaction, the nucleophilic addition still happens and then followed elimination which produces water. The first example of this type of reaction is the reaction of carbonyl with alcohol to form acetal.
As you notice from the reaction above, the reaction is catalysed by acid and it is a reversible reaction. Besides that, more precisely the reaction requires a dry acid catalyst because in the presence of aqueous acid will lead into acetal hydrolysis. However, the reaction also produces water molecules and this problem can be overcome by removing water such as using azeotropic distillation or molecular sieves. The mechanism of this reaction is shown below.
 |
| Acetal formation |
As stated before the reaction requires dry acids, so an organic acid can be used such as p-toluenesulphonic acid (TsOH) and the reason of the organic acid is it can be dissolved in typical organic solvents used for acetal formation.
Acetal is very important in our life because one of our energy sources is in the form of acetal. This energy source is sucrose. Besides that, other sugars such as glucose and fructose is in form of hemiacetal.
Furthermore, the formation of hemiacetal of glucose is shown below.
 |
| The mechanism of acetal formation of glucose |
Another similar reaction is with thiols, instead of alcohols, to form thioacetal. Besides that, the acid in this case is a lewis acid (e.g. BF3). Moreover, this reaction is important to form an alkane from carbonyl compound.
The third example is the reaction with primary amines in acid condition to give imines. As mentioned before, this reaction happens in acid condition which means there is a problem, which is the amines itself is basic, so it might be protonated. Therefore, in this reaction pH control is important and it was found that the optimum pH for this reaction is 5. Furthermore, in pH 5 the amines are still be protonated but it still have a sufficient amount to be the nucleophile.
 |
| The relative rate of reaction of imines formation as a function of pH |
From the previous part (see
here) up to this point, we should be able to see the nucleophile that is compatible with acid. The nucleophile such as alcohols, thiols, and amines are compatible with acid; meanwhile organolithium, organomagnesium, and borohydride are not compatible with acid because they generate nucleophile which is very strong base.
Hence, the mechanism of this reaction is shown below.
 |
| Imines formation |
As you notice from the mechanism above, the attack of amines into carbonyl does not require protonated carbonyl because amines is better nucleophile than alcohols or water. Furthermore, imines are the precursor compound to form secondary amines by using mild reducing agent such as NaB(CN)H3, sodium cyanoborohydride.
When a carbonyl compound reacts with secondary amines it would give different product from reaction with primary amines. This reaction would produces enamines, the compound has conjugated amines with alkene. The reaction and its mechanism are shown below.
 |
| Enamines formation |
With same mechanism reaction as shown earlier, it can react with several primary amine derivates.
 |
| Reaction of acetone with some primary amine derivates |
All the products from reactions above give unique melting points depends on the carbonyl compound, so it can be used to determine the carbonyl compound without spectroscopy.
















Comments