The Chemistry of Solar Energy
In this section, we will try to discuss the chemistry behind the solar cell such as the types of photovoltaic cell and how it works. Furthermore, we will also see the future application.
To begin with, we will start from the Sun as the energy source for photovoltaic cell. The sun is comprised mainly of H and He by 74% (w/w) and 25% (w/w) respectively. The energy from the sun is produced by the nuclear fusion reaction. At the core of the sun, H-2 (deuterium) and H-3 (tritium) undergo nuclear fusion reaction to form He-4, a neutron, and as well the energy itself, and the reaction is:
Moreover, the energy that is produced from H fusion reaction is 17.6 MeV (1 eV = 96.5 kJ). Besides that, the sun also acts as a black-body radiator at a temperature of 5800 °C, and it is emitting photons mostly in visible range. A black body radiator can be defined as an idealised object that emits a temperature-dependent spectrum of light and a photon is the elementary particle responsible for electromagnetic phenomena. Moreover, the sun emits photon mainly at visible range and the consequence of this matter is different wavelength of the light carries different energy, in figure below it is shown the solar radiation spectrum.
Then, from the solar radiation can come what we call as solar energy. Solar energy is the technology of obtaining useful energy from sunlight and it has been used for many centuries in a number of applications:
The electrical conductivity from a semiconductor compound is based on the band gap of the compound. The band gap can be defined as the energy gap between valence band and conduction band as shown in figure below.
A pure semiconductor (intrinsic) contains just the right amount of electrons to fill the valence band. Then, impurities can be added to either introduce electrons into conduction band, or remove electrons in valence band; and the process is known as 'doping'. Generally, there are 2 type 'doped' semiconductor, n-type and p-type. A semiconductor can be called the n-type semiconductor if the current is carried by negatively charged electrons, the example of n-type is the small quantities of P atom is added to melt before Si crystal is formed. In the other sides, the p-type semiconductor is the type which is the current is carried by electron holes, and for example if a small quantities of B atom added to melt before Si crystal is formed.
We can move to general structure of photovoltaic cells. The cell basically consists of two thin semiconductor wafers; one 'n-type' and one 'p-type' semiconductor. Then, two wafers are placed together to create a point of contact which is know as 'p-n junction. The imbalance in electrical charge at the p-n junction creates electric field and the electric field acts as a diode.
After it is placed together, the cell is irradiated by light, so the electrons are excited. Hence, the excited electrons flow toward positive charge and the electron hole pairs are formed.
Last step is charge recombination via external circuit. The electric field prevents charge recombination across p-n junction and the electron flow provides current, and the electric field supplies voltage. Moreover, the production of a voltage difference across a p-n junction is based on the photovoltaic effect.
Most of the photovoltaic cell (PV cell), crystalline Si is the most prevalent material used for solar cells, by far. Moreover, Si doping as we have seen in the earlier section by doping it with B atom or P atom. Besides that, amorphous Si is also used, to reduce the production costs. A 6 cm diameter Si solar cell can produce a current of approximately 0.5 A and a potential of approximately 0.5 V. To increase the potential and the current of PV cell, cells can be connected in series to form modules (of cells) and arrays (of modules).
Because Si band gap has specific energy (1.1 eV - 1127 nm), not all the solar radiation spectrum can be absorbed to generate electricity. Hence, to measure the efficiency of PV cell, one of the parameter is quantum efficiency which refers to the percentage of absorbed photons that produce electron-hole pairs. The range of quantum efficiency is from 6% for amorphous based solar cell up to 40% for multiple-junction research lab cells. However, higher efficiency is not necessarily higher economical value. Furthermore, variety of thin-film technologies are being developed. In later on, we will see briefly certain type of PV cell that is commonly used in these days.
One of the example of thin-film technologies, CdTe (cadmium-tellurium) PV cell. The advantage of this PV cell is easy to make thin films, and can be delivered on a large scale. However, it has disadvantage of potential toxic as Cd metal is used.
Besides thin-film technologies, a multi-junction cells are also used to convert a higher proportion of the sun's energy to electricity, and the example is GaAs (Galium-Arsenic) multi-junction. The advantage of this cell is it has high quantum efficiency, up to 40% and it is designed to absorb most of the energy from the solar spectrum, but the problem of this cell is expensive. Hence, it is used for special applications, such as satellitess and space exploration.
Then, there is type of PV cell that is called Dye-Sensitised Solar Cells (DSSCs). DSSCs are photoelectrochemical cells that utilise the photo-sensitization of wide band-gap semiconductor and it was in 1991 by Michael Grätzel. Moreover, the current DSSC has quantum yields around 11%, but considerably cheaper than Si-based solar cells. The dye molecules that are commonly used for DSSC are typically Ru-bhased polypyridyl complexes
Moreover, improvements in light-harvesting capacity and stability of dye molecules is the major focus in development of technology.
The electricity generation in DSSC basically has 4 main steps. Firstly, light is absorbed the dye (S), and high energy excited state is formed (S*). After excitation, electron is transferred to the conduction band (CB) of the semiconductor (process of injection) and the electric field allows extraction of electron to anode. Then, transfer of electron from redox mediator (A-) to the dye to stabilised positive charge that is formed. Lastly, the electrons are transfered from anode to cathode, though external load, to close the circuit and return redox mediator (A) to its reduced form. Moreover, maximum cell potential corresponds to the difference between the Fermi level of the semiconductor and redox potential of the mediator and DSSC in someway mimic photosynthesis.
To begin with, we will start from the Sun as the energy source for photovoltaic cell. The sun is comprised mainly of H and He by 74% (w/w) and 25% (w/w) respectively. The energy from the sun is produced by the nuclear fusion reaction. At the core of the sun, H-2 (deuterium) and H-3 (tritium) undergo nuclear fusion reaction to form He-4, a neutron, and as well the energy itself, and the reaction is:
Moreover, the energy that is produced from H fusion reaction is 17.6 MeV (1 eV = 96.5 kJ). Besides that, the sun also acts as a black-body radiator at a temperature of 5800 °C, and it is emitting photons mostly in visible range. A black body radiator can be defined as an idealised object that emits a temperature-dependent spectrum of light and a photon is the elementary particle responsible for electromagnetic phenomena. Moreover, the sun emits photon mainly at visible range and the consequence of this matter is different wavelength of the light carries different energy, in figure below it is shown the solar radiation spectrum.
Then, from the solar radiation can come what we call as solar energy. Solar energy is the technology of obtaining useful energy from sunlight and it has been used for many centuries in a number of applications:
- Heat (e.g hot water, cooking)
- Electricity generation (photovoltaic cells, heat engines)
- Chemical processes (desalination of sea water, photoelectrochemical cells)
| PS10 (foreground) and PS20 (background) Solar Power Plant |
Photovoltaics and Photovoltaic cells
Photovoltaic cells (also called solar cells) convert light energy (photon) into electrical energy and it works under the principle of photovoltaic effect of semiconductors which is used to generate electricity. The photovoltaic effect can be described as the production of a voltage difference across a p-n junction (we will see that later) as a result of the absorption of photons and it was discovered in 1839 by French experimental physicist, Edmund Becquerel.| Alexandre-Edmond Becquerel |
A pure semiconductor (intrinsic) contains just the right amount of electrons to fill the valence band. Then, impurities can be added to either introduce electrons into conduction band, or remove electrons in valence band; and the process is known as 'doping'. Generally, there are 2 type 'doped' semiconductor, n-type and p-type. A semiconductor can be called the n-type semiconductor if the current is carried by negatively charged electrons, the example of n-type is the small quantities of P atom is added to melt before Si crystal is formed. In the other sides, the p-type semiconductor is the type which is the current is carried by electron holes, and for example if a small quantities of B atom added to melt before Si crystal is formed.
We can move to general structure of photovoltaic cells. The cell basically consists of two thin semiconductor wafers; one 'n-type' and one 'p-type' semiconductor. Then, two wafers are placed together to create a point of contact which is know as 'p-n junction. The imbalance in electrical charge at the p-n junction creates electric field and the electric field acts as a diode.
After it is placed together, the cell is irradiated by light, so the electrons are excited. Hence, the excited electrons flow toward positive charge and the electron hole pairs are formed.
Last step is charge recombination via external circuit. The electric field prevents charge recombination across p-n junction and the electron flow provides current, and the electric field supplies voltage. Moreover, the production of a voltage difference across a p-n junction is based on the photovoltaic effect.
Most of the photovoltaic cell (PV cell), crystalline Si is the most prevalent material used for solar cells, by far. Moreover, Si doping as we have seen in the earlier section by doping it with B atom or P atom. Besides that, amorphous Si is also used, to reduce the production costs. A 6 cm diameter Si solar cell can produce a current of approximately 0.5 A and a potential of approximately 0.5 V. To increase the potential and the current of PV cell, cells can be connected in series to form modules (of cells) and arrays (of modules).
Because Si band gap has specific energy (1.1 eV - 1127 nm), not all the solar radiation spectrum can be absorbed to generate electricity. Hence, to measure the efficiency of PV cell, one of the parameter is quantum efficiency which refers to the percentage of absorbed photons that produce electron-hole pairs. The range of quantum efficiency is from 6% for amorphous based solar cell up to 40% for multiple-junction research lab cells. However, higher efficiency is not necessarily higher economical value. Furthermore, variety of thin-film technologies are being developed. In later on, we will see briefly certain type of PV cell that is commonly used in these days.
One of the example of thin-film technologies, CdTe (cadmium-tellurium) PV cell. The advantage of this PV cell is easy to make thin films, and can be delivered on a large scale. However, it has disadvantage of potential toxic as Cd metal is used.
Besides thin-film technologies, a multi-junction cells are also used to convert a higher proportion of the sun's energy to electricity, and the example is GaAs (Galium-Arsenic) multi-junction. The advantage of this cell is it has high quantum efficiency, up to 40% and it is designed to absorb most of the energy from the solar spectrum, but the problem of this cell is expensive. Hence, it is used for special applications, such as satellitess and space exploration.
Then, there is type of PV cell that is called Dye-Sensitised Solar Cells (DSSCs). DSSCs are photoelectrochemical cells that utilise the photo-sensitization of wide band-gap semiconductor and it was in 1991 by Michael Grätzel. Moreover, the current DSSC has quantum yields around 11%, but considerably cheaper than Si-based solar cells. The dye molecules that are commonly used for DSSC are typically Ru-bhased polypyridyl complexes
| Common dye molecules structure |
The electricity generation in DSSC basically has 4 main steps. Firstly, light is absorbed the dye (S), and high energy excited state is formed (S*). After excitation, electron is transferred to the conduction band (CB) of the semiconductor (process of injection) and the electric field allows extraction of electron to anode. Then, transfer of electron from redox mediator (A-) to the dye to stabilised positive charge that is formed. Lastly, the electrons are transfered from anode to cathode, though external load, to close the circuit and return redox mediator (A) to its reduced form. Moreover, maximum cell potential corresponds to the difference between the Fermi level of the semiconductor and redox potential of the mediator and DSSC in someway mimic photosynthesis.
Fermi level = Energy of the highest filled state at T is 0 KFurthermore, photoelectrochemical cells can be used to generate hydrogen from water and the mechanisms are:
- Irradiation of dye results in charge separation, with electron in semiconductor and "holes" in the dye (S+).
- In acidic solution (electrolyte), water is oxidised by the "holes" at the anode.
- Electrons is generated at anode transferred via external circuit to cathode.
- Oxygen is produced at anode, and hydrogen at cathode. Hence, the overall reaction is:
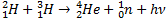















Comments