In this section, we will see how the sunscreen lotions provide a protective barrier from UV radiation and as well the chemical component of sunscreen lotions. To start with, we will have a brief look of the electromagnetic wave spectrum.
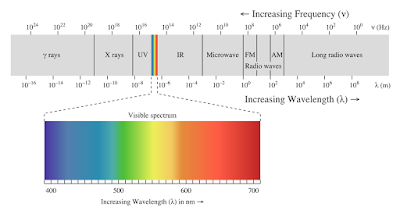 |
| Electromagnetic spectrum |
With regards to sunscreen lotions, we will focus on the ultraviolet (UV) radiation, especially UV-A, UV-B, and UV-C. As shown in figure above, there are 4 main regions of UV radiations, UV-A (315 - 400 nm), UV-B (290 - 315 nm), UV-C (200-290 nm), and UV-V (100 - 200 nm). Moreover, as the wavelength is going longer, the energy of the spectrum is decreasing. Hence, an attractive suntan is due to the generation of a protective chemical, melanin, which screens out part of the UV radiation. Moreover, this is the body's attempt to protect itself from potential skin damage.
Firstly, a slightly energetic radiation of UV-C makes a slightly problem, but UV-C does not penetrate the ozone layer. However, this problem can be a huge problem as the ozone hole is being created due to pollution. Secondly, the less energetic radiation, UV-B, cannot pass through window glass but it can cause sunburn and tanning. In the other sides, it helps the body to produce vitamin D. The intensity of UV-B is varies with weather, which is more intense in summer, midday, and at high altitudes and near equator. Then, UV-B radiation comprises 0.5% of the sun rays. The other problem that can be caused by UV-B is it causes more than 90% of non-melanoma skin cancers as well implicated in cataract formation. Lastly, the least energetic, UV-A, it can pass through window glass and the intensity is not affected by changes in altitude or weather. Hence, it present all day, every day of the year, and it comprises 5% of the sun's rays. UV-A radiation can penetrates deep into skin layers and it can cause long-term skin damage or perhaps cancer in the worst case.
As we saw the description above, UV-B radiation can cause damage to the genetic code. This is caused by the DNA absorbs UV-B radiation via its pyrimidine groups, so it disrupts its molecular structure. This defects mean that genetic code cannot be 'read' properly, so it can get mutated proteins and cell death. Moreover, it can lead to skin burns, blistering, and skin cancer. In the other sides, cells have evolved the ability to repair the damaged DNA. It helps by certain enzymes that remove damaged DNA and replace it with the correct sequence (using information located elsewhere on the DNA). Hence, this makes the DNA more resilient to UV-B radiation damage.
How Sunscreen Lotions Work?
Generally, suntan lotions block UV-B selectively and letting UV-A produce a slow tan. In the other sides, sunscreen lotions are formulated to block both types of UV radiation. Besides that, protection level are shown by
sun protection factor (SPF) which is related to the percentage of UV-B radiation that penetrates to the skin but it does not tell about the degree of protection from UV-A. For example, if the SPF is 2, so only 50% UV-B radiation penetrates, thus you can stay in the sun twice longer. Moreover, if the SPF is 33, it means only 3% UV-B radiation penetrates, so you can stay in the sun 33 times longer. However, UV-A is the UV radiation that can penetrate deeply into skin, so it generates free radicals and inhibits body repair mechanism. Hence there is a question about the importance to block UV-A as UV-B protection.
Full spectrum sunblocks absorb UV-A and UV-B using multiple organic aromatic compounds that are selected for their water resistance, hypoallergenicity, cost effectiveness, specific wavelength, and the other factors. Moreover, it also can use ultrafine inorganic oxide sunscreens.
Generally, there are 2 basic types of sunscreen lotions, organic molecule-based and inorganic particle-based. In traditional organic molecule-based sunscreens, it has narrow spectrum, so 3 or more components is required for SPF. Besides that, it should be readily absorbed into skin since it is molecular components. However, it can cause allergic reactions and eye irritation, it is known to suffer from photo-instability, and it need to formulate with solvents, so increasing the skin irritation risk.
In the other sides, inorganic particle-based sunscreens provides broad spectrum coverage by using ultrafine parlticles to block entire UV spectrum. Besides that, it is not absorbed into skin since particles are too large, do not cause allergic reactions, and it does not need to be formulated with solvents.
Organic-based molecules sunscreen lotions
The mechanism protection of 2 types of sunscreen is very different from each other. In organic-based UV absorbers, it absorbs UV radiation. Therefore, it needs a very high UV extinction coefficient (
ε) and also good photo-stability. The UV extinction coefficient is based on the Beer-Lambert's Law as shown below.
 |
The inorganic oxide-based particles sunscreen lotion protection mechanism (left) and
Beer-Lambert's law (right) |
Where, A is the absorbance,
l is the length of light pathway (in this case is the thickness of sunscreen layer), [
C] is the concentration, and
ε is the UV extinction coefficient. Hence, SPF increases with ε, [C], and l.
 |
| Common organic UV absorber compounds |
In organic-based UV absorbers, there 4 common organic molecules that is used in the formulation. Firstly is octyl methoxycinnamate which absorbs strongly in UV-B range but it is not waterproof. Hence, it is used in combination with waterproof ingredients such as octyl salicylate. Octyl salicylate absorbs radiation over the entire UV-B range but it has relatively low extinction coefficient. Moreover, it is phot-stable, hypoallergenic, and waterproof. Thirdly is oxybenzone which absorbs both UV-A and UV-B, but generally considered as a UV-A blocker. Lastly is dioxybenzone which also absorbs both UV-A and UV-B, but generally considered as a UV-B blocker. Hence, we can see the mechanism of protection of this organic molecule and for example is oxybenzone.
 |
| The reaction of oxybenzone when it absorbs UV radiation |
The internal hydrogen bonding in oxybenzone provides efficient dissipation mechanism for absorbed UV and it absorbs at 300 - 400 nm. When oxybenzone absorbs the UV radiation, it rearranges to from UV-excited non-aromatic state, and it backs to ground states by releasing heat. Moreover, appropriate choice of substituent allows optimisation of absorption envelope (range) and similar molecules are also used for UV stabilisation of various 'exterior use' plastics.
 |
| A cartoon of skin topography |
Besides that, the flow properties (rheology) of sunscreen can affect the value of SPF. Generally, human skin has a relatively rough topography on a micron length scale. Hence, the 'runny' sunscreen must be avoided, since bare skin is exposed to UV radiation, and this problem is due to non-optimised sunscreen rheology.
 |
| The condition when the skin is not fully covered with sunscreen lotion |
For example, if a sunscreen has SPF is 10, so transmission (T) is 0.10 for 100% skin coverage. However, if there are only 90% coverage the SPF is:
Inorganic oxide-based particles sunscreen lotions
In inorganic oxide-based, it form the sunscreen layer to scatter the UV radiation, so it requires a high refractive index. Besides that, consumers prefer transparent lotion, so the particles need to be small particles size. Hence, it is a good example of modern nanotechnology.
 |
| The inorganic oxide-based particles sunscreen lotion protection mechanism |
Generally, there are 2 oxides that commonly used in the formulation,
ultrafine TiO2 and ultrafine ZnO. Ultrafine
TiO2 particles absorbs UV-B and scatter UV-A radiation. In the other sides, ultrafine ZnO particles absorb both UV-A and UV-B radiation.
 |
A plot of wavelength against the relative attenuation in arbitrary units for certain UV absorbers.
Click the figure to enlarge it. |
From the figure above, it is shown that ZnO offer superior protection compared to
TiO2 due to wider absorption wavelength band. However, these data were provided by ZnO manufacturer, so it is less reliable. In the other sides, the data from Oxonica (Oxford, UK)
TiO2 is twice as effective as ZnO per unit mass.
 |
The ultrafine ZnO (left) and a plot of mean particle diameter of ZnO and differential number distribution.
Click to enlarge the picture. |
| |
The control size of ZnO particle size is also important and it offers 'broad spectrum' UV protection and excellent photostability. Moreover, ZnO nanoparticles should be provided as as a surface-treated. Hence, smaller ZnO particles absorb more UV to give more transparent formulation, which consumers prefer. L'Oreal & Boots uses inorganic
TiO2 and ZnO pigment in combination with organic absorbers for most effective sunscreens.
Beside the control size, the surface pre-treatment is also important for inorganic oxide. In fact, UV irradiation of inorganic oxides can generate free radical in situ, so surely
TiO2 and ZnO nanoparticles should not be used in sunscreen at all is the main question. An experiment from USA showed 105 nm ZnO nanoparticles are passivated by a PDMS surface layer and this converted into a silica layer on heating to 400 °C. Besides that, from Optisol, Oxonica showed that 20 nm TiO
2 nanoparticles are passivated by addition of less than 1% Mn atoms. This impurity prevents free radical formation and also mops up free radicals created by the in situ decomposition of organic sunscreen compounds.
 |
| Rohm and Haas' Sun SpheresTM |
Besides that, hollow latex particles can also be used as UV scatterers. As we saw in
the chemistry of paint, the use of hollow latex particles in paint formulations to scatter visible light. The same principle can be used for sunscreens, but smaller latex particles (d = 200 nm) is required to scatter UV radiation which is shorter in wavelength, compared to visible light.











Comments