The Chemistry of Hydrogen Fuel: Economy, Generation, and Storage
The supply of clean and renewable energy is probably one of the greatest challenges facing humanity in the 21st century and mainly the energy sources is came from fossil fuel. It is estimated that current reserves will be exhausted in approximately 50 years, so it is vital that alternative energy sources are found soon. This is in part due to the finite supply of oil, but also to reduce harmful emissions associated with the burning of oil. One of the alternative energy source is hydrogen.
The hydrogen economy is a hypothetical future in which energy is stored as hydrogen. The energy can be released either directly (e.g. burning in a car combustion engine) or by conversion to alternvative energy sources. Unlike fossil fuels, hydrogen does not emit any greenhouse gases upon combustion.
Significant problems to be overcome in the generation and storage of hydrogen for it to become a useful alternative. Nowadays, the energy density by volume of hydrogen fuel is still lower than existing energy sources such as fossil fuel, but it has relatively high density by mass. This is caused by hydrogen is not so dense, so it has low energy density by volume. In the other sides, because hydrogen is so light, the energy density by mass is relatively higher than fossil fuel.
The hydrogen production in the world scale is around 65 million metric tons are produced annually and the economic value of hydrogen produced in 2005 around $135 billion per year. There are currently 2 main uses of hydrogen today:
1. About half is used in the production of ammonia via the Haber-Bosch process. Ammonia is used directly or indirectly as fertilizer.
2. The other half is used in hydrocracking, which is a process to convert heavy hydrocarbons into light hydrocarbons suitable for use as fuels.
Hydrogen can be produced in either laboratory scale or in industrial scale. In laboratory, hydrogen can be produced by reaction of a metal (e.g. Zn) with acid.
Besides that, hydrogen can be prepared by reacting Al with aqueous acid or base.
Alternative methods are used for industrial preparation
In industrial scale, the source of hydrogen is mainly come from natural gas and oil which is cover almost 80%. Besides that, solar energy can be used to generate hydrogen using bioreactors or photoelectrochemical cells. In this section we will discuss 2 main process of hydrogen production in industry, steam reforming and electrolysis.
The condition of this reaction is required catalyst (typically either a Ni, Co, alkali metal or rare earth metal mixture) at high pressure (up to 40 atm or 40 000 kPa) and high temperature (up to 960 °C). The product mixture is known as syngas.
To improve the overall efficiency of the reaction, the water gas shift reaction is employed.
The reaction is performed in two steps:
Step 1: High temperature reaction with steam. A catalyst such as Fe3O4 or other transition metal oxides are required.
Step 2: Low temperature reaction with steam. The catalyst is typically a mixture of Cu, Zn, or Al.
Besides methane, reforming the other hydrocarbons, notably oil products, can be achieve with similar reactions.
Similar methods are used to generate hydrogen from coal, although partial oxidation of the coal with O2 is used in addition to steam. Moreover, steam methane reforming is the most common method of producing commercial bulk hydrogen. Then, the disadvantages and the advantages of steam reforming are:
The reactions that occurs in the hoffmann voltameter are:
Hence, the overall reaction is:
However, pure water is a very poor conductor of electricity, so strong acid such as H2SO4 are used as electrolytes to carry the current and close the circuit. Besides that, electrolysis of pure water requires excess energy in the form of overpotential to overcome activation barriers.
The electricity supplied for electrolysis can come from renewable sources such as hydropower or wind turbines. Currently, power obtained from these sources tends to be too expensive as it requires approximately 50 kW hours to electricity per kg of H2 formed. After can be produced, the next problem is how it is stored and transported.
Besides that, hydrogen gas is not very dense, even at high pressure (e.g. at 81 000 kPa, it has density about 40 kg m-3, compare with water = 1 000 kg m-3). This phenomena is caused by the low polarisability of H2, that results in very weak intermolecular (van der Waals) interaction. However, compressed hydrogen gas can be used for energy sources by using fuel cells.
Besides as a gas, hydrogen also can be stored as liquid. Liquid hydrogen has properties such as boiling point at 20.93 K (-252.76 °C) and density about 70.8 kg m-3 at atmospheric pressure. Moreover, compressing and chilling hydrogen to its liquid state uses about 30% of the energy the hydrogen is storing. Part of this energy is consumed because of a quantum mechanical phenomena, nuclear spin.
Because hydrogen has single electron, so it can produce different spin which implies to magnetic moment. There are 2 possibility of spin at hydrogen molecules, ortho (when it has parallel spin) or para (when it has different spin). At room temperature, hydrogen consist of approximately 75% ortho and 25% para, but at cryogenic temperatures para-H2 is the most stable form. Ortho-para conversion is highly exothermic, and occurs on a timescale of days. Hence, spin conversion to para-H2 is carried out during liquefaction over catalysts of iron oxide, hydroxide, or chromium oxide supported on alumina.
Therefore, there are a number of disadvantages to using liquid hydrogen to store and transport hydrogen. The disadvantages are:
Besides it can be stored in pure condition, either in gas or liquid state, hydrogen can also be stored as materials-based storage. There are 3 generic mechanisms for material-based hydrogen storage:
Firslty, hydrogen can be stored as metal hydrides. Metal hydrides can store and release hydrogen at close to ambient temperatures and pressures. Hydrogen reacts with many transition metals and their alloys to form metal hydrides (MHn). The hydrogen storage in metal hydrides is an absorption process, with the hydrogen atoms often occupying interstitial sites within the metal lattice. Interstitial lattice can be defined as a position between the regular positions in an array of atoms or ions that can be occupied by other atoms or ions. To form metal hydrides, it is required to react with the electropositive elements, such as Sc, Yt, lanthanides, actinides, and members of the Ti and Va groups. Using transition metals means that the hydrogen gravimetric density is typically less than 3 %(w/w) H. Moreover, hydrogen gravimetric density is the mass of hydrogen in a compound, expressed as a percentage.
Besides metal hydrides, it can be stored as complex hydrides with group 1, 2, and 4 light metals (e.g. Li, Mg, and Al), give rise to a large variety of metal-hydrogen complexes. The lighter metals have potential for higher gravimetric hydrogen capacities than the metal hydrides. The main difference in comparison to metal hydrides is the transition to an ionic or covalent compound.
Alanates (AlH4-) have shown potential as hydrogen storage materials. The reactions are:
The first reaction is catalysed using titanium dopant and it becomes thermodynamically favourable at temperature above 33 °C, and the second reaction takes place at higher temperature (110 °C). Importantly, the reaction is reversible and the starting material can be regenerated at 270 °C under 175 bar hydrogen pressure in 2-3 hours.
Besides alanates, LiBH4 can store hydrogen and it has the highest known gravimetric mass density at room temperature [18% (w/w)H]. The reaction of LiBH4 is:
Moreover, the reaction takes place upon melting point at 280 °C.
As we have seen, complex hydrides show significant advantages over metal hydrides:
Thirdly, carbon-based materials show the ability to store hydrogen as well. Single-walled carbon nanotubes are being studied as hydrogen storage materials (adsorption). The published gravimetric capacities are in the range 3-10 % (w/w) at room temperature, but there is some controversy with these results due to difficulties in reproducing the data.
Besides carbon nanotubes, high surface area microporous metal-organic frameworks (MOFs) have received a lot of attention recently as materials for the adsorption of hydrogen. MOFs with hydrogen gravimetric capacities of up to 7.5% (w/w) at 77 K have been developed.
Lastly, chemical reaction can store hydrogen as result of the reaction. First reaction is hydrogenation/ dehydrogenation reaction and these reactions have been studied for many years as a means of hydrogen storage. One of the example is conversion of decalin into naphtalene as shown below.
The reaction can release 7.3% (w/w) H at 210 °C and a platinum-based or noble metal supported catalyst is required to enhance the kinetics of hydrogen evolution.
Then, a new chemical approach involves hydrogen generation from ammonia borane that has a hydrogen storage potential of up to 20 %(w/w) H.
Hydrogen is released simply by heating the compound and the first reaction takes place at temperatures above 110 °C and it has 6.1% (w/w) H. Then, the second reaction occurs at about 155 °C [6.5% (w/w) H] and the third reaction releases the remaing hydrogen possible, but occurs at temperature higher than 500 °C.
Another reaction is the hydrolysis reactions that involve metals and chemical hydrides with water to produce hydrogen atom. One of the example is ammonia borane as starting material and the reaction is:
This reaction occurs at room temperature with Co, Ni, or Cu catalyst with 8.9% (w/w) H. Besides that, sodium borohydrides, NaBH4,can generate hydrogen from hydrolysis reaction.
The main advantages of this reaction is:
- The gaseous hydrogen is produced only when needed.
- The fuel is non-flammable; at ambient pressure and temperature.
- The reaction occurs at low reaction temperature (60 - 80 °C)
Moreover, a number of companies have developed systems for commercial application of this reaction.
From all technologies that we have seen for hydrogen storage, in table below shows the current status of hydrogen storage technologies.
Moreover, in figure below is the recent target from the United States' Department of Energy.
To sum up, there are a few future challenges of hydrogen generation. Firstly, cheap and renewable sources of required hydrogen for any future "hydrogen economy". Secondly, overarching technical challenge for transport applications is to store required hydrogen for conventional driving range (> 300 miles). Lastly, requirements for other technologies may be generally less restrictive, but improvements are still needed in current technology.
The hydrogen economy is a hypothetical future in which energy is stored as hydrogen. The energy can be released either directly (e.g. burning in a car combustion engine) or by conversion to alternvative energy sources. Unlike fossil fuels, hydrogen does not emit any greenhouse gases upon combustion.
Significant problems to be overcome in the generation and storage of hydrogen for it to become a useful alternative. Nowadays, the energy density by volume of hydrogen fuel is still lower than existing energy sources such as fossil fuel, but it has relatively high density by mass. This is caused by hydrogen is not so dense, so it has low energy density by volume. In the other sides, because hydrogen is so light, the energy density by mass is relatively higher than fossil fuel.
The hydrogen production in the world scale is around 65 million metric tons are produced annually and the economic value of hydrogen produced in 2005 around $135 billion per year. There are currently 2 main uses of hydrogen today:
1. About half is used in the production of ammonia via the Haber-Bosch process. Ammonia is used directly or indirectly as fertilizer.
2. The other half is used in hydrocracking, which is a process to convert heavy hydrocarbons into light hydrocarbons suitable for use as fuels.
Hydrogen can be produced in either laboratory scale or in industrial scale. In laboratory, hydrogen can be produced by reaction of a metal (e.g. Zn) with acid.
Besides that, hydrogen can be prepared by reacting Al with aqueous acid or base.
Alternative methods are used for industrial preparation
 |
| The Alternative Method of Hydrogen Production for Industrial Preparation |
Hydrogen Production
1. Steam reforming
Steam reforming is currently the main process used in the industrial production of hydrogen. A mixture of methane and water vapour is used.The condition of this reaction is required catalyst (typically either a Ni, Co, alkali metal or rare earth metal mixture) at high pressure (up to 40 atm or 40 000 kPa) and high temperature (up to 960 °C). The product mixture is known as syngas.
To improve the overall efficiency of the reaction, the water gas shift reaction is employed.
The reaction is performed in two steps:
Step 1: High temperature reaction with steam. A catalyst such as Fe3O4 or other transition metal oxides are required.
Step 2: Low temperature reaction with steam. The catalyst is typically a mixture of Cu, Zn, or Al.
Besides methane, reforming the other hydrocarbons, notably oil products, can be achieve with similar reactions.
Similar methods are used to generate hydrogen from coal, although partial oxidation of the coal with O2 is used in addition to steam. Moreover, steam methane reforming is the most common method of producing commercial bulk hydrogen. Then, the disadvantages and the advantages of steam reforming are:
a. Disadvantages
- The reforming reaction takes place at high temperatures, making it slow to start up and requiring costly high temperature materials.
- Sulphur compounds present in the fuel and CO produced can poison certain catalysts.
- The catalyst is frequently very expensive.
- Uses natural resources as feedstock, and produces CO2 gas.
b. Advantages
- Relatively cheap technology.
- Advanced technology and infrastructure in comparison to other hydrogen technologies.
- Fuel cell vehicles using hydrogen produced from natural gas reduce greenhouse gas emissions by 60%
2. Electrolysis
Electrolysis of water decomposes water into oxygen and hydrogen gas with the aid of an electric current. Electrolysis cell consist of two electrodes submerged in an aqueous electrolyte solution and connected to a direct current source. One of the earliest design for an apparatus for the electrolysis of water is a hoffmann voltameter and often used as a small-scale electrolytic cell.| Hoffmann Voltameter |
Hence, the overall reaction is:
However, pure water is a very poor conductor of electricity, so strong acid such as H2SO4 are used as electrolytes to carry the current and close the circuit. Besides that, electrolysis of pure water requires excess energy in the form of overpotential to overcome activation barriers.
The electricity supplied for electrolysis can come from renewable sources such as hydropower or wind turbines. Currently, power obtained from these sources tends to be too expensive as it requires approximately 50 kW hours to electricity per kg of H2 formed. After can be produced, the next problem is how it is stored and transported.
Hydrogen Storage
There are a few problems concerning about hydrogen generation, especially how to store it. As most of us know, pure hydrogen can only be stored as a gas at room temperature and the critical temperature of hydrogen is 33 K (-240 °C). The critical temperature can be defined as the temperature above which a gas cannot be liquefied, no matter how much pressure is applied, and the phase diagram for hydrogen is shown below.| Phase Diagram of Hydrogen |
 |
| London City Bus and Honda FCX Clarity which are powered by compressed hydrogen gas |
 |
| Hydrogen spin |
Therefore, there are a number of disadvantages to using liquid hydrogen to store and transport hydrogen. The disadvantages are:
- Large amount of energy required to liquefy hydrogen.
- Liquefaction is an expensive process.
- It has to be stored at 20 K, and has a fast boil off rate.
- It requires large insulated storage tanks.
 |
| BMW Hydrogen 7, powered by liquid hydrogen |
- Absorption: Hydorgen is absorbed directly into the bulk of the material.
- Adsorption: Hydrogen is physi- or chemi-sorbed onto a material or liquid surface.
- Chemical reaction: Hydrogen generation anda storage take place via chemical reactions.
Firslty, hydrogen can be stored as metal hydrides. Metal hydrides can store and release hydrogen at close to ambient temperatures and pressures. Hydrogen reacts with many transition metals and their alloys to form metal hydrides (MHn). The hydrogen storage in metal hydrides is an absorption process, with the hydrogen atoms often occupying interstitial sites within the metal lattice. Interstitial lattice can be defined as a position between the regular positions in an array of atoms or ions that can be occupied by other atoms or ions. To form metal hydrides, it is required to react with the electropositive elements, such as Sc, Yt, lanthanides, actinides, and members of the Ti and Va groups. Using transition metals means that the hydrogen gravimetric density is typically less than 3 %(w/w) H. Moreover, hydrogen gravimetric density is the mass of hydrogen in a compound, expressed as a percentage.
Besides metal hydrides, it can be stored as complex hydrides with group 1, 2, and 4 light metals (e.g. Li, Mg, and Al), give rise to a large variety of metal-hydrogen complexes. The lighter metals have potential for higher gravimetric hydrogen capacities than the metal hydrides. The main difference in comparison to metal hydrides is the transition to an ionic or covalent compound.
Alanates (AlH4-) have shown potential as hydrogen storage materials. The reactions are:
The first reaction is catalysed using titanium dopant and it becomes thermodynamically favourable at temperature above 33 °C, and the second reaction takes place at higher temperature (110 °C). Importantly, the reaction is reversible and the starting material can be regenerated at 270 °C under 175 bar hydrogen pressure in 2-3 hours.
Besides alanates, LiBH4 can store hydrogen and it has the highest known gravimetric mass density at room temperature [18% (w/w)H]. The reaction of LiBH4 is:
Moreover, the reaction takes place upon melting point at 280 °C.
As we have seen, complex hydrides show significant advantages over metal hydrides:
- It has greater hydrogen capacity
- It is increased uptake and release kinetics
- It has lower cost
- Relatively new field, whole range of compounds need to be explore.
Thirdly, carbon-based materials show the ability to store hydrogen as well. Single-walled carbon nanotubes are being studied as hydrogen storage materials (adsorption). The published gravimetric capacities are in the range 3-10 % (w/w) at room temperature, but there is some controversy with these results due to difficulties in reproducing the data.
Besides carbon nanotubes, high surface area microporous metal-organic frameworks (MOFs) have received a lot of attention recently as materials for the adsorption of hydrogen. MOFs with hydrogen gravimetric capacities of up to 7.5% (w/w) at 77 K have been developed.
Lastly, chemical reaction can store hydrogen as result of the reaction. First reaction is hydrogenation/ dehydrogenation reaction and these reactions have been studied for many years as a means of hydrogen storage. One of the example is conversion of decalin into naphtalene as shown below.
The reaction can release 7.3% (w/w) H at 210 °C and a platinum-based or noble metal supported catalyst is required to enhance the kinetics of hydrogen evolution.
Then, a new chemical approach involves hydrogen generation from ammonia borane that has a hydrogen storage potential of up to 20 %(w/w) H.
Hydrogen is released simply by heating the compound and the first reaction takes place at temperatures above 110 °C and it has 6.1% (w/w) H. Then, the second reaction occurs at about 155 °C [6.5% (w/w) H] and the third reaction releases the remaing hydrogen possible, but occurs at temperature higher than 500 °C.
Another reaction is the hydrolysis reactions that involve metals and chemical hydrides with water to produce hydrogen atom. One of the example is ammonia borane as starting material and the reaction is:
This reaction occurs at room temperature with Co, Ni, or Cu catalyst with 8.9% (w/w) H. Besides that, sodium borohydrides, NaBH4,can generate hydrogen from hydrolysis reaction.
- The gaseous hydrogen is produced only when needed.
- The fuel is non-flammable; at ambient pressure and temperature.
- The reaction occurs at low reaction temperature (60 - 80 °C)
Moreover, a number of companies have developed systems for commercial application of this reaction.
 |
| Daimler Chrysler Na Car, which is powered by hydrolysis of NaBH4 |
To sum up, there are a few future challenges of hydrogen generation. Firstly, cheap and renewable sources of required hydrogen for any future "hydrogen economy". Secondly, overarching technical challenge for transport applications is to store required hydrogen for conventional driving range (> 300 miles). Lastly, requirements for other technologies may be generally less restrictive, but improvements are still needed in current technology.
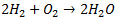















Comments