The Chemistry of Battery
Most of the portable electronic devices in these day use battery as the power source. In this section, we will see how chemistry is applied into our daily life through battery.
Battery is already known for a long time ago and the earliest battery was dated around 250 BC and 640 AD. It was found by Wilhelm Konig in 1938, but no one known the exact application. Many scientists suggest it might be used for electroplating.
Generally, a battery is a device that stores chemical energy and makes it available in electrical form. From this basis, the function of battery itself. After the baghdad battery, voltaic cells (also known as galvanic cells) were invented after 18th century by Italian scientists, Alessandro Volta and Luigi Galvani.
Basically, batteries could contain one or more voltaic cells and voltaic cells are electrochemical cells that can generate an electric current. Volta demonstrated that electricity did not come from the animal tissue (as Galvani stated) but rather from the different metals (brass and iron) coming into moist contact with each other. This device that demonstrated the Volta's idea is known as the voltaic pile.
In general, a voltaic cell consists of:
1. Two separate compartment called "half cells", each containing an electrode.
2. Each electrode is made of different metals.
3. Electrolyte solutions that neutralise charges that build up in solution. Hence an electrolyte solution should have ability to conduct electricity (mainly an ionic salt solution).
In 1836, John Frederick Daniell developed a voltaic cell which used zinc and copper with the solutions of their ions.
In voltaic cell (or daniell cell) works based on the redox (reduction-oxidation) reaction. In each half cell, it might occur either reduction or oxidation reaction. The electrode at which oxidation occurs is called the anode (negative terminal). In the other sides, the electrode at which reduction occurs is called the cathode (positive terminal). Moreover, in daniell cell, Zn loses electrons more readily than copper. Hence, Zn will be oxidised and Cu(II) ions will be reduced, and the half reactions are:
From the reactions above, we can say that the mass of anode is reduced as Zn is oxidised, whilst mass of the anode increases as the half reaction produced Cu metal. To ensure there is the electric current, a porous barrier is placed to separate the half cells. The porous barrier is used to ensure the ions flows along the system to neutralise the charges. Moreover, a porous barrier in daniell cell is required to allow sulphate ions to flow through, but block Zn(II) and Cu(II) ions. Hence, a concomitant flow of sulphate ions in solution from cathode side to anode side. Then, the electrons will flow from the anode to cathode through the external circuit and the movement of sulphate ions through the electron flow in the external circuit. Therefore, the overall cell reaction of daniell cell is:
The amount of energy per unit charge from the voltaic cell is called the cell potential (electromotive force) of the cell and it is express in volts and it is related to the difference potential energy between the two electrodes. The value of cell potential may predicted using standard electrode potentials for the two metals. As we saw in daniell cell, the cell reactions split into 2 half-reactions representing the reaction at the cathode and anode. Hence, the cell potential (emf) can be calculated using:
It would be helpful to be able to work out oxidation and reduction potentials for electrodes in order to calculate the cell potential for any cell.
Based on the standard electrode potential, there are 2 main problems that need to overcome. Firstly, the electrode potential cannot be determined in isolation, but in reaction with some other electrode. Hence, to address this problem is by measuring potentials with respect to a standard hydrogen electrode. Therefore, the standard electrode potential for H is set as 0 V. Secondly, the electrode potential depends on concentration, temperature, and pressure in the case of a gas electrode and to overcome is by using standard thermodynamic conditions for measurement (the concentration of solute is 1.00 M at 1 atm pressure and 298 K). In the table below is shown for the value of standard potential for certain reactions.
From the data above, the bigger the standard reduction potential, the easier it is to be reduced. From the table above, by applying our equation to calculate the cell potential, the value for daniell cell can be calculated as 1.10 V by assuming it happens on standard thermodynamic condition. However, a real voltaic cell is unlikely to be used under standard thermodynamic conditions.
The Nernst equation can be used to calculate the real cell potential under working conditions and the equation is:
Where, R is the gas constant (8.31451 J mol-1 K-1), n is the number of electrons transfered, T is the temperature in Kelvin (K), F is Faraday's constant (9.648 53 x 104 C mol-1), and Q is the thermodynamic reaction quotient. The reaction quotient is the same as an equilibrium constant expression, but for partial pressure or concentrations of the reactants and products before the system reaches equilibrium. Hence, for the reaction below,
The value for Q is:
For example in daniell cell, the reaction is:
Since Zn and Cu metal is a solid, so it can be assumed the concentration is constant. Hence, Q for daniell cell is:
The C-Zn battery has the voltage around 1.5 V and this battery has some problems that can lead to leakage and shelf-life of around 1.5 years. The problems are:
1) As the battery is used the Zn case becomes thinner.
2) Electrolyte solution can leak from the battery reducing the amount of ammonium ions available.
3) Zn case get thinner even when battery is not being used because ammonium chloride is acidic.
4) Hydrogen can surround the cathode increasing internal resistance.
To overcome most of the problem, alkaline batteries can be used to address the problem associated with C-Zn batteries by using KOH as an electrolyte. Hence, the half reactions are:
Hence, the overall cell reaction is:
Moreover, the advantages of the alkaline batteries are:
1) They produce the same voltage as a C-Zn battery.
2) They avoid using Zn corroding ammonium ions and do not form any gaseous products.
3) They have much longer shelf-life than C-Zn batteries
From the reaction above, we can spot the reaction is the equilibrium reaction with forward reaction as discharge reaction. In NiCd battery, Ni(OH) acts as cathode, Cd acts as anode, and the electrolyte is typically KOH. Moreover, the electrolyte is not consumed in this battery and the cell voltage is 1.20 V, which is slightly lower than for alkaline batteries.
Where, MH is the metal hydride which acts as anode and M is the metal. Ni-MH has 2-3 times the capacity of a Ni-Cd battery of an equivalent size.
Basically, the Li-ion battery works almost the same way with another batteries. Both cathode and anode contain Li ions between each layers. When the battery is connected to external circuit, electrons flow from anode to cathode. Then, to compensate with the charge different, Li ion travel from anode to cathode in the electrolyte through a separator. In the other way, when it is charged by connecting to power supply, the electrons move from cathode to anode and the Li ions will travel from cathode to anode in electrolyte solution in similar way when the battery is discharged.

The reaction in this cell is:
Hence, the overall reaction is:
Moreover, the standard electromotive of Li ion battery is 3.70 V.
To compare of 2 rechargeable batteries (Ni-MH batteries and Li ion), we can see the advantages of each batteries. The advantages of Ni-MH batteries are:
a. It is cheaper than Li ion batteries.
b. Cell capacity diminishes more slowly, so it is longer lasting than Li ion batteries.
c. Compatible with devices that use alkaline AA batteries.
d. Safer than Li ion batteries
Meanwhile, the advantages of Li ion batteries are:
a. Li ion batteries have a higher energy density than NiMH, which means Li ion batteries are lighter than Ni-MH batteries.
2. It has lower self-discharge rate than Ni-MH batteries (Li ion: 5-10% per month, Ni-MH = ~30% per month)
3. It is more environmentally friendly than Ni-MH, which is due to its component. In Ni-MH batteries use rare earth metals which is less environmentally safe.
4. It has less memory effect, which means it has little loss of voltage over lifetime.
Lead-acid battery was invented in 1859 by French physicist Gaston Planté. Nowadays, lead-acid batteries have the ability to provide high current. Hence, it is ideal for use in cars to provide the high current for starter motors and also it requires little maintenance.
To summarise our discussion about the batteries, batteries remain a convenient source of electricity for a number of application. However, batteries are expensive energy sources that also use hazardous chemical in their manufacture and consume valuable resources. As the age is going, technological advances mean that portable electronic devices require more and more power. Nowadays, alternatives to traditional battery technologies are being investigated.
Battery is already known for a long time ago and the earliest battery was dated around 250 BC and 640 AD. It was found by Wilhelm Konig in 1938, but no one known the exact application. Many scientists suggest it might be used for electroplating.
| Schematic diagram of Baghdad Battery |
Generally, a battery is a device that stores chemical energy and makes it available in electrical form. From this basis, the function of battery itself. After the baghdad battery, voltaic cells (also known as galvanic cells) were invented after 18th century by Italian scientists, Alessandro Volta and Luigi Galvani.
 |
| Alessandro Giuseppe Antonio Anastasio Volta (left) and Luigi Aloisio Galvani (right) |
 |
| The voltaic pile |
1. Two separate compartment called "half cells", each containing an electrode.
2. Each electrode is made of different metals.
3. Electrolyte solutions that neutralise charges that build up in solution. Hence an electrolyte solution should have ability to conduct electricity (mainly an ionic salt solution).
In 1836, John Frederick Daniell developed a voltaic cell which used zinc and copper with the solutions of their ions.
 |
| Daniell cell |
From the reactions above, we can say that the mass of anode is reduced as Zn is oxidised, whilst mass of the anode increases as the half reaction produced Cu metal. To ensure there is the electric current, a porous barrier is placed to separate the half cells. The porous barrier is used to ensure the ions flows along the system to neutralise the charges. Moreover, a porous barrier in daniell cell is required to allow sulphate ions to flow through, but block Zn(II) and Cu(II) ions. Hence, a concomitant flow of sulphate ions in solution from cathode side to anode side. Then, the electrons will flow from the anode to cathode through the external circuit and the movement of sulphate ions through the electron flow in the external circuit. Therefore, the overall cell reaction of daniell cell is:
The amount of energy per unit charge from the voltaic cell is called the cell potential (electromotive force) of the cell and it is express in volts and it is related to the difference potential energy between the two electrodes. The value of cell potential may predicted using standard electrode potentials for the two metals. As we saw in daniell cell, the cell reactions split into 2 half-reactions representing the reaction at the cathode and anode. Hence, the cell potential (emf) can be calculated using:
It would be helpful to be able to work out oxidation and reduction potentials for electrodes in order to calculate the cell potential for any cell.
Based on the standard electrode potential, there are 2 main problems that need to overcome. Firstly, the electrode potential cannot be determined in isolation, but in reaction with some other electrode. Hence, to address this problem is by measuring potentials with respect to a standard hydrogen electrode. Therefore, the standard electrode potential for H is set as 0 V. Secondly, the electrode potential depends on concentration, temperature, and pressure in the case of a gas electrode and to overcome is by using standard thermodynamic conditions for measurement (the concentration of solute is 1.00 M at 1 atm pressure and 298 K). In the table below is shown for the value of standard potential for certain reactions.
From the data above, the bigger the standard reduction potential, the easier it is to be reduced. From the table above, by applying our equation to calculate the cell potential, the value for daniell cell can be calculated as 1.10 V by assuming it happens on standard thermodynamic condition. However, a real voltaic cell is unlikely to be used under standard thermodynamic conditions.
The Nernst equation can be used to calculate the real cell potential under working conditions and the equation is:
Where, R is the gas constant (8.31451 J mol-1 K-1), n is the number of electrons transfered, T is the temperature in Kelvin (K), F is Faraday's constant (9.648 53 x 104 C mol-1), and Q is the thermodynamic reaction quotient. The reaction quotient is the same as an equilibrium constant expression, but for partial pressure or concentrations of the reactants and products before the system reaches equilibrium. Hence, for the reaction below,
The value for Q is:
For example in daniell cell, the reaction is:
Since Zn and Cu metal is a solid, so it can be assumed the concentration is constant. Hence, Q for daniell cell is:
After we discussed about the basic electrochemistry as the foundation for batteries' knowledge, so we can move to batteries itself. Basically, batteries can be divided into two main types; rechargeable and non-rechargeable (disposable). For non-rechargeable batteries are also called primary cells and used once than discarded. The reason why this battery can be used only once is due to the chemical reactions involved are not easily reversible or sometimes non-reversible. In the other sides, the rechargeable batteries are commonly called secondary cells, it can be re-charged once drained by passing electric current through the cells. Next, we will discuss some examples of common primary and secondary cells.
A. Carbon-Zinc (C-Zn) battery
| Georges Leclanché's C-Zn battery |
C-Zn battery was invented in 1866 by Georges Leclanché an is often called the Leclanché cell. This type of cell is a primary cell or disposable one. In the 20th century, it was the most common battery used in daily life and now it is still the cheapest primary batteries. This batteries are often used by manufacturers when devices such as remote controls, flaslights, toys, or transistor radios have batteries included. Moreover, this battery is known as a dry cell because there is no free liquid present, so the electrolyte is in the form of moist paste. In this picture below is the inside of C-Zn battery.
The anode of this cell is the Zn case and the half reaction is:
Then, the cathode is C surrounded by manganese(IV) oxide and the half reaction is slightly more complicated than the anode. Firstly the ammonium ions in the electrolyte react at the cathode to generate hydrogen gas.
The hydrogen formed reacts with Mn(IV) oxide and reduces it to Mn(III) oxide.
Hence, the overal reaction is:The C-Zn battery has the voltage around 1.5 V and this battery has some problems that can lead to leakage and shelf-life of around 1.5 years. The problems are:
1) As the battery is used the Zn case becomes thinner.
2) Electrolyte solution can leak from the battery reducing the amount of ammonium ions available.
3) Zn case get thinner even when battery is not being used because ammonium chloride is acidic.
To overcome most of the problem, alkaline batteries can be used to address the problem associated with C-Zn batteries by using KOH as an electrolyte. Hence, the half reactions are:
Hence, the overall cell reaction is:
Moreover, the advantages of the alkaline batteries are:
1) They produce the same voltage as a C-Zn battery.
2) They avoid using Zn corroding ammonium ions and do not form any gaseous products.
3) They have much longer shelf-life than C-Zn batteries
B. The rechargeable batteries
The rechargeable batteries are also called secondary cells and the electrochemical reactions are reversible allowing the battery to be recharged. It can be achieved by applying a voltage from an external charging source that reverses the cell reaction. As with primary cells, there are a number of types employed, commonly nickel-cadmium (NiCd), nickel-metal hydride (NiMH) and lithium ion (Li-ion).1. Nickel-cadmium (NiCd) batteries
The chemical reaction which occurs in NiCd battery is:From the reaction above, we can spot the reaction is the equilibrium reaction with forward reaction as discharge reaction. In NiCd battery, Ni(OH) acts as cathode, Cd acts as anode, and the electrolyte is typically KOH. Moreover, the electrolyte is not consumed in this battery and the cell voltage is 1.20 V, which is slightly lower than for alkaline batteries.
2. Nickel-metal hydride batteries
The metal in a nickel-metal hydride battery is typically an intermetallic compound (e.g. AB5 A: rare earth mixture of La, Ce, Nd, Pr; B = Ni, Co, Mn, and/or Al). The reaction of this cell is:Where, MH is the metal hydride which acts as anode and M is the metal. Ni-MH has 2-3 times the capacity of a Ni-Cd battery of an equivalent size.
3. Lithium ion batteries
In this battery, lithium cobalt oxide acts as the cathode and the anode is carbon compound, which graphite is used, and the electrolyte is LiPF6 or LiBF4 in organic solvent. Lithium cobalt oxide consists of layer of Li (purple spheres) that lie between slabs formed by cobalt and oxygen atoms (red and blue spheres) as shown in picture below.| Lithium cobalt oxide |
Basically, the Li-ion battery works almost the same way with another batteries. Both cathode and anode contain Li ions between each layers. When the battery is connected to external circuit, electrons flow from anode to cathode. Then, to compensate with the charge different, Li ion travel from anode to cathode in the electrolyte through a separator. In the other way, when it is charged by connecting to power supply, the electrons move from cathode to anode and the Li ions will travel from cathode to anode in electrolyte solution in similar way when the battery is discharged.

The reaction in this cell is:
Hence, the overall reaction is:
Moreover, the standard electromotive of Li ion battery is 3.70 V.
To compare of 2 rechargeable batteries (Ni-MH batteries and Li ion), we can see the advantages of each batteries. The advantages of Ni-MH batteries are:
a. It is cheaper than Li ion batteries.
b. Cell capacity diminishes more slowly, so it is longer lasting than Li ion batteries.
c. Compatible with devices that use alkaline AA batteries.
d. Safer than Li ion batteries
Meanwhile, the advantages of Li ion batteries are:
a. Li ion batteries have a higher energy density than NiMH, which means Li ion batteries are lighter than Ni-MH batteries.
2. It has lower self-discharge rate than Ni-MH batteries (Li ion: 5-10% per month, Ni-MH = ~30% per month)
3. It is more environmentally friendly than Ni-MH, which is due to its component. In Ni-MH batteries use rare earth metals which is less environmentally safe.
4. It has less memory effect, which means it has little loss of voltage over lifetime.
C. Lead-acid batteries
 |
| Gaston Planté and his original design of lead-acid battery |
Lead-acid battery was invented in 1859 by French physicist Gaston Planté. Nowadays, lead-acid batteries have the ability to provide high current. Hence, it is ideal for use in cars to provide the high current for starter motors and also it requires little maintenance.
 |
| Lead-acid battery in discharge condition |
A lead acid battery consists of a Pb metal anode and PbO2 cathode with 6M sulphuric acid as the elctrolyte. The chemical reactions which occurs in the cell is:
Hence, the overall discharge reaction and the standard potential cell is:
As the reaction occurs, the size of both the anode and cathode will decrease as battery is used.
In a car battery, the electrode plates are actually lead grills which are used to increase the surface area of the electrode. The standard 12 V car battery contains 6 lead-acid cell joined together and the electrodes in the cell are separated by a permeable membrane. Moreover, to recharge the battery, the charges are reversed by applying an external power source and the reaction will be reversed.
 |
| Lead-acid battery in charging condition |











.png)


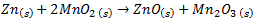




Comments