Clathrate compound
 A clathrate, clathrate compound or cage compound is a chemical substance consisting of a lattice of one type of molecule trapping and containing a second type of molecule. The name clathrate complex used to refer only to the inclusion complex of hydroquinone, but recently it has been adopted for many other weak composites which consist of a host molecule (forming the basic frame) and a guest molecule (held in the host molecule by inter-molecular interaction). Clathrates are also called host-guest complexes, inclusion compounds, and adducts (chiefly in the case of urea and thiourea). They used to be called molecular compounds.
A clathrate, clathrate compound or cage compound is a chemical substance consisting of a lattice of one type of molecule trapping and containing a second type of molecule. The name clathrate complex used to refer only to the inclusion complex of hydroquinone, but recently it has been adopted for many other weak composites which consist of a host molecule (forming the basic frame) and a guest molecule (held in the host molecule by inter-molecular interaction). Clathrates are also called host-guest complexes, inclusion compounds, and adducts (chiefly in the case of urea and thiourea). They used to be called molecular compounds.A clathrate hydrate, in particular, is a special type of gas hydrate in which a lattice of water molecules encloses molecules of a trapped gas. Large amounts of methane naturally frozen in this form have been discovered both in permafrost formations and under the ocean sea-bed. Researchers have begun to investigate silicon and germanium clathrates for possible semiconducting and superconducting properties.
The word clathrate is derived from the Latin clatratus meaning with bars or a lattice
History
The history of clathrate compounds is relatively recent. Clathrate hydrates were discovered in 1810 by Humphry Davy. Clathrates were studied by P. Pfeiffer in 1927 and in 1930, E. Hertel defined "molecular compounds" as substances decomposed into individual components following the mass action law in solution or gas state. In 1945, H. M. Powell analyzed the crystal structure of these compounds and named them clathrates.
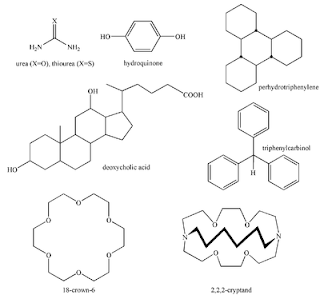
Urea- and thiourea-hosted clathrates were applied to the separation of paraffin. Thereafter, cyclodextrin, crown ether, and cryptand were found as host molecules (see figure). A much studied host molecule is Dianin's compound.
Properties
Clathrate complexes are various and include, for example, strong interaction via chemical bonds between host molecules and guest molecules, or guest molecules set in the geometrical space of host molecules by weak intermolecular force. Typical examples of host-guest complexes are inclusion compounds and intercalation compounds.
Clathrates can be isolated as chemically different species, and may have structural and positional isomers (enantiomers and diastereomers).
Comments