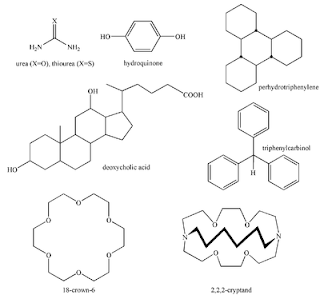
Introduction of International Year of Chemistry 2011

The International Year of Chemistry 2011 ( IYC 2011) is a worldwide celebration of the achievements of chemistry and its contributions to the well-being of humankind. Under the unifying theme “ Chemistry—our life, our future ,” IYC 2011 will offer a range of interactive, entertaining, and educational activities for all ages. The Year of Chemistry is intended to reach across the globe, with opportunities for public participation at the local, regional, and national level. The goals of IYC2011 are to increase the public appreciation of chemistry in meeting world needs, to encourage interest in chemistry among young people, and to generate enthusiasm for the creative future of chemistry. The year 2011 will coincide with the 100th anniversary of the Nobel Prize awarded to Madame Marie Curie—an opportunity to celebrate the contributions of women to science. The year will also be the 100th anniversary of the founding of the International Association of Chemical Societies, pr...